2017上海普陀区高三二模化学试题及答案
- 格式:doc
- 大小:117.50 KB
- 文档页数:6

上海市普陀区2015届高三化学第二次模拟考试试题(扫描版)普陀区2014学年第二学期高三质量调研化学试卷参考答案及评分标准2015.4一、选择题(本题共10分,每小题2分,只有一个正确选项)1 2 3 4 5D C D B B6 7 8 9 10 11 12 13 14 15 16 17A A CB B D BC BD C C三、选择题(本题共20分,每小题4分。
只有一个正确选项的,多选不给分;有两个正18 19 20 21 22AC A AC BD AB四、(本题共12分)23、2:3;0.6NA (1分×2)24、溶液变蓝;(1分)5I- + IO3 - +6 CH3COOH →3I2 + 3H2O + 6CH3COO-(2分)25、c(H+) c(ClO-)/c(HClO) ;(1分)10-7.5 或3.16×10-8(1分)26、大于(2分)27、差;(1分) 温度升高,(溶解)平衡①逆向移动,Cl2(aq)浓度减少,使得(化学平衡)②逆向移动,c(HClO) 减少,(杀菌效果变差。
) (2分)五、(本题共12分)28、; (2分)29、C (1分)、 Na2SiO3 + CO2 + H2O→Na2CO3+H2SiO3↓ 合理即可。
(1分)30、ac (2分)31、(1)ad (2分);(2)(0.16/t)mol/(L.min)(2分); 0.32(1分)32、正(1分)六、(本题共12分)33.缺少温度计;大试管接触烧杯底部(或其他合理说法)。
(2分)34.使苯与混酸混合均匀;能及时移走反应产生的热量(或其他合理答案)。
(2分)35.浅黄色;硝基苯、苯(2分)。
b 、d (2分)36.②氢氧化钠溶液;分液漏斗 ③水洗、分离或水洗 ⑤蒸馏。
(2分)37.(1)温度过高引起硝酸的分解、苯的挥发。
(2)温度过高,有其他能溶于硝基苯的产物生成,或“有副反应,生成其他产物”等合理答案。

普陀区2016学年高三年级第二次学业质量调研测试英语学科试卷(时间120分钟,满分140分)考生注意:I.本试卷共12页。
满分140分。
考试时间120分钟。
2.答题前,考生务必在答题卡(纸)上用钢笔或水笔清楚填写姓名、准考证号,并用铅笔正确涂写准考证号。
3.答案必须全部涂写在答题卡(纸)上。
第1-20小题,第31-70小题,均由机器阅卷,考生应将代表正确答案的小方格用铅笔涂黑。
注意试题题号和答题纸编号一一对应,不能错位。
答案需要更改时,必须将原选项擦去,重新选择。
答案不能涂写在试卷上,涂写在试卷上一律不给分。
第21-30小题,第IV, V大题(即第72-75小题)和VI大题,其答案用钢笔或水笔写在答题纸上,如用铅笔答题或写在试卷上也一律不给分。
I. Listening Comprehension(略)II. Grammar and VocabularySection ADirections: After reading the passage below, fill in the blanks to make the passage coherent and grammatically correct. For the blanks with a given word, fill in each blank with the proper form of the given word; for the other blanks, use one word that best fits each blank.Wildlife in DeclineThe populations of Earth’s wild vertebrates (脊椎动物)have declined by 58% over the past four decades, according to the Living Planet Report 2016 published by the World Wildlife Fund.Climate change and activities such as deforestation and poaching(偷猎)are in large part (21)______(blame) for the decline. If the trend continues, by 2020, the world (22)________(lose) two-thirds of its vertebrate biodiversity. “Sadly, there is no sign yet (23)________ this rate will decrease,” the report says.“Across land, fresh water and the oceans, human activities are forcing wildlife populations to the edge," says Marco Lambertini, director-general of WWF International.The Living Planet Report is published every two years. It aims to provide an assessment of the state of the world’s wildlife. The 2016 study included 3700 different species of birds, fish, mammals, amphibians and reptiles around the world. The team collected data from more than 3000 sources, including government statistics and surveys (24) ______ (carry) out by conservation groups. They then analyzed (25) ______ the population sizes had changed over time.Lambertini said some groups of animals had done worse than others. ''We do see particularly strong declines (26) ______ the freshwater environment. For freshwater species alone, the decline stands at 81% since 1970. This is related to the way that water (27)________(use) and taken out of freshwater systems, and also to the fragmentation(分裂)of freshwater systems through dam building, for example.”The report also highlighted other species, such as African elephants, (28) ________ nave suffered huge declines in recent years, and sharks, which are threatened by overfishing.(29) ________ ________ ________ all the terrifying facts, however, some conservationists say there is still hope. “One of the things that I think is the most important is that these wild animals haven't yet gone extinct,” said Robin Freeman,head of the Zoological Societ y of London. “On the whole, (30) ________ are not dying out, andthat means we still have opportunities to do something about the decline.”Section BDirections: Fill in each blank with a proper word chosen from the box. Each word can be used only once. Note that there is one word more than you need.My job puts me in contact with extraordinary leaders in many fields. So I tend to ____31____ a lot on leadership and how we can inspire successful teamwork, cooperation, and partnerships. In my experience, it is clear that the most successful leaders—both men and women—always demonstrate three ____32____ traits.TrustworthinessLeaders must set an example of honesty and justice and earn the trust of their teams through their everyday actions. When you do so with positive energy and enthusiasm for ____33____ goals and purpose, you can deeply connect with your team and customers. A culture of trust enables you to empower employees and ____34____ the foundation for communication, accountability, and continuous improvement.Compassion (共情)You can't forget that organizational success ____35____ from the hearts and minds of the men and women you lead. Rather than treating your people as you’d like to be treated, treat them as they would like to be treated. Small gestures like choosing face-to-face meetings or sending personal ____36____ can have an enormous impact on the spirits of the teams. In addition to thanks and praise, you must also understand people’s needs, pressures, and individual goals, which will allow you to lead them more effectively and ____37____ to their personal ambitions and professional development.DecisivenessIn times of ____38____ employees long for clarity. As a leader, you won't always have all of the answers—no one expects you to—so you must be open to listening and learning from others. Once you understand a particular challenge and ____39____ the options, you have to be confident in making bold and optimistic decisions.Successful leadership demands a lifelong commitment to sharpening these three basic skills. Wherever you have the opportunity to ____40____, the qualities of trustworthiness, compassion, and decisiveness are the keys to leadership and organizational success.Boxing is a popular sport that many people seem to be fascinated by. Newspapers, magazines and sports programmes on TV frequently ____41____ boxing matches. Professional boxers earn a lot of money, and successful boxers are ____42____ as big heroes.It seems to me that some people, especially men, find it ____43____ because it is an aggressive sport. When they watch a boxing match, they can t ____44____ the winning boxer, and this gives them the feeling of being a t ____45____ themselves. It is a fact that many people have feelings of aggressionIII. Reading ComprehensionSection ADirections: For each blank in the following passage there are four words or phrases marked A, B, C and D. Fill in each blank with the word or phrase that best fits the context.Boxing is a popular sport that many people seem to be fascinated by . Newspapers , magazines and sports programmes on TV frequently _________boxing matches . Professional boxers earn a lot of money , and successful boxers are _______as big heroes.It seems to me that people , especially men ,find it _______because it is an aggressive sport . When they watch a boxing match , they can _______ the winning boxer , and this gives them the feeling of being a ______ themselves . It is a fact that many people have feeling of aggression from time to time , but they cannot show their _______in their everyday lives . Watching a boxing match gives them an outlet for this aggression .However , there is a ______side to boxing . It can be a very dangerous sport . Although boxers wear gloves during the fights , and amateur boxers ______have to wear helmets , there have frequently been accident in both professional and amateur boxing , sometimes with ________consequences . Boxers have suffered from head injuries , and occasionally , fighters have even been killed as a result of being knocked out in the__________. Furthermore , studies have shown that there are often long-term effects of boxing , in the form of serious brain _______,even if a boxer has never been knocked out .I am personally not at all in ______of aggressive sports like boxing . I think it would be better if less time was _______to aggressive sports on TV, and we welcomed more men and women from non-aggressive sports as our heroes and heroines in our society . I believe that the world is aggressive enough already ! Of course , people like _______sports , and so do I , but I think that ______other people in an aggressive way is not something that should be regarded as a sport.41. A. broadcast B. cover C. host D. design42. A. kept B. individual C. thought D. treated43. A. appealing B. subjective C. violent D. challenging44. A. pick up B. believe in C. identify with D. long for45. A. winner B. spectator C. inspector D. trainer46. A. ambition B. aggression C. energy D. strength47. A. positive B. indifferent C. deadly D. negative48. A. otherwise B. somehow C. even D. barely49. A. dramatic B. eye-catching C. emotional D. special50. A. court B. ring C. pitch D. yard51. A. loss B. drain C. damage D. disorder52. A. favour B. process C. charge D. power53. A. shifted B. transformed C. given D. delivered54. A. competitive B. quiet C. cooperative D. regular55. A. invading B. insulting C. teasing D. hittingSection BDirections:Read the following three passages. Each passage is followed by several questions or unfinished statements. For each of them there are four choices marked A. B, C and D. Choose the one that fits best according to the information given in the passage you have just read.(A)FrankensteinFrankenstein was a book by Mary Shelly ----it’s been adapted for the screen dozens of times. The story of Frankenstein is told through a series of letters written by Captain Robert Walton to his sister , as he leads an expedition (长征)to the North Pole . On the way , he meets Victor Frankenstein , who tells Walton the story of his life. Frankenstein is the sur name of the guy who creates the monster . The monster doesn’t actually have a game . Anyway , Victor is a scientist who’s desperate to discover the secret of life . After years of study , he makes an enormous creature out of human remains and brings it to life . Victor intends it to be beautiful . Unfortunately , the creature turns out really hideous , and Victor runs away in terror . Although the monster is good and kind , humans are scared of it . When they mistreat it , the monster becomes angry and evil . Wanting revenge on its creator , the monster murders Dr. Frankenstein’s brother , his wife , and his best friend . When Victor figures out the monster is behind all the deaths , he swears to track it down and kill it .This book was written in 1816, right after a period called the Enlightenment . The Enlightenment emphasized the pursuit of knowledge and reason , and gave rise to the scientific method . Mary Shelley criticized the Enlightenment through the character of Victor Frankenstein , “ He is a neg ative example of an Enlightenment scientist -------he pursues knowledge at any cost , and his obsession with discovering the secret of life destroys him , as well as his friends and family .” Some Enlightenment thinkers might have seen such a loss as neces sary for the advancement of science , but not Mary Shelley . She and her husband , poet Percy Shelly , were part of the Romanic Movement in art and literature . Romancism was a reacrion against the Enlightenment’s embrace of rationality and reason . The Romantics emphasized emotion over rationality , and thought people should feel awe and terror in regard to nature . Frankenstein incorporates all these ideas. To Shelley , Frankenstein doesn’t fear and respect the world of nature enough ------she says that by tempering with nature , he brings about complete disaster . Frankenstein is not just a great Romantic novel . It’s also considered one of the first major works of science fiction . It influences a whole generation of writers , and the monster has become one of the most recognizable figures in Western culture.56. Which of the following is closest in the meaning to ‘hideous’ in Paragraph 1?A. UnattractiveB. EngagingC. CharmingD. Handsome57. What is Victor Frankenstein’s fatal weakness?A. His love of scienceB. His rejection of his own creationC. His lack of respect for natureD. His inability to form human relationship58. How was the Romantic era different from the Enlightenment ?A. The Romantic era emphasized emotion ; the Enlightenment emphasized reason .B. The Romantic era occurred during the 20th century ; the Enlightenment occurred during the 19th century .C. The Romantic era emphasized poetry ; the Enlightenment emphasized prose .D. The Romantic era saw major scientific discoveries ; the Enlightenment was an era of literary discovery .59. What effect did “ F rankenstein” have on later works of fiction ?A. It inspired books about the EnlightenmentB. It inspired technical writingC. It inspired books of poetryD. It inspired science-fiction writing60. If you are a 22-year-old nurse , you can apply for the railcard without ________.A. the signature of your director B $ 28c. application form D. passport-sized photos61. The 1/3 OFF discount may not apply for the railcard holders who travel at _______.A. 11 pm on Sunday in AugustB. 7. am on Tuesday in FebruaryC. 7 am on Monday in JulyD. 11 pm on Friday in March62. Which of the following is True according to the leaflet ?A. If you railcard doesn’t have your name signed , it will be used by someone else.B. The benefits of a railcard are transferable to your friend of your age .C. If you have no ticket but have boarded a train , you will still be eligible for a discounted ticketD. If railcard holders wish to use the Eurostar network , they must pay the full fare.The ‘ Phone Stack(堆)’GameWhenever Michael Carl , the fashion market director at Vanity Fair , goes out to dinner with friends , he plays something, called the “ phone stack” game : Everyone places their phones in the middle of the table ; whoever looks at their device before the check arrives picks up the bill . As smartphones continue to burrow(钻入) their way into our lives , and wearable devices like Google Glass threaten to eat into our person space even further , overburdened users are carving out their own device-free zones with special tricks and life hacks .“Disconnecting is a luxury that we all need ,” Lesley M. M. Blume , a Ne w York writer keeps her phone away from the dinner table at home .” The expectation that we must always be available to employers ,colleague, family : It creates a real obstacle in trying to set aside private time . But that private time is more important than ever. “ Much of the digital detoxing (戒毒)is centered on the home , where urgent e-mails from co-workers , texts from friends , Instagram photos from acquaintances and updates on Facebook get together to disturb domestic quietness.A popular method is to appoint a kind of cellphone lockbox , like the milk tin that Brandon Holley , the former editor of lucky magazine , uses. “ If my phones is buzzing or lighting up , it’s still a distraction , so it goes in the box . “, said Ms. Holley , who lives in a r ow house in Red Hook , Brooklyn , with her son ,Smith , and husband , John .” It’s not something I want my kid to see.” Sleep is a big factor , which is why some people draw the cellphone-free line at the bedroom.” I don’t want to sleep next to something t hat is a charged ball of information with photos an e-mails ,” said Peter Som , the fashion designer , who keeps his phone plugged in the living room overnight .” “It definitely is a head clearer and describes daytime and sleep time clearly .”Households with young children are especially mindful about being overconnected , with parents sensitive to how children may imitate bad habits . But it’s not just inside the home where users are separating themselves from the habit . Cellphone overusers are making efforts to disconnect in social settings ,whether at the request of the host or in the form of friendly competition . The phone-stack game is a lighthearted way for friends to police against rude behavior when eating out . The game gained popularity after Brian Perez, a dancer in Los Angeles , posted the idea online.63. What might be the reason for Michael Carl to play the “ phone stack” game?A. His friends aren’t willing to pay for the meal voluntarily .B. He wants to do some funny things with those phonesC. He has been fed up with digital devices being present everywhereD. The wearable devices have brought threats to his privacy .64.Why is it difficulty for people to break away from their digital device at home ?A. Because they have to do some work at homeB. Because they are expected to be always available to the outsideC. Because people have been addicted to digital devices.D. Because digital devices can enrich people’s family life.65. What does Peter Som do to ensure his sleeping quality at night ?A. He puts his phone in the living room .B. He ignores any information in the phoneC. He deletes all information in his phoneD. He puts his phones in a lockbox66. Why does the phone-stack game become popular as soon as it is posted online?A. The game helps create a harmonious relationship among friends.B. The game makes the host get along well with the guestC. The game can prevent children from imitating their parents’ behaviorD. The game meets people’s demand for keeping away from phones easilySection CDirections: Read the following passage. Fill in each blank with a proper sentence given in the box. Each sentence can be used only once. Note that there are two more sentences than you need.“Any apple today ?”, Effie asked cheerfully at my window ,. I followed her to her truck and bought a kilo . On credit , of course . Cash was the one thing in the world I lacked just them .All pretense (借口)of payment was drooped when our funds , food and fuel decreased to alarming lows. Effie came often , always bringing some gift: a jar of peaches or some firewood . There were other generosities.___________Effie was not a rich woman . Her income , derived from investment she had made while running an interior decorating shop , had never exceeded $200 a month , which she supplemented by selling her apples .But she always managed to help someone poorer .Years passed before I was able to return the money Effie had given me from time to time . She was ill now and had aged rapidly in the last year .” Here , darling , “ I said , “ is what I owe you ,” _____________” Give it back as I gave it to you -----a little at a time.” “ I think she believed there was magic in the slow discharge of a love debt.The simple fact is that I never repaid the whole amount to Effie , for she died a few weeks later . By now , the few dollars Effie gave me have been multiplied many times . But a curious thing began to happen .___________At that time , it seemed that my debt would forever go unsettled . So the account can never be marked closed , for Effie’s love will go on in hearts that have never known her .IV. Summary WritingDirections:Read the following passage. Summarize the main idea and the main point(s) of the passage in no more than 60 words. Use your own words as far as possible.Chaco Great HouseAs early as the twelfth century A.D., the settlements of Chaco Canyon in New Mexico in the American Southwest were notable for their "great houses," massive stone buildings that contain hundreds of rooms and often stand three or four stories high. Archaeologists have been trying to determine how the buildings were used. Whilethere is still no universally agreed upon explanation, there are three competing theories.One theory holds that the Chaco structures were purely residential, with each housing hundreds of people. Supporters of this theory have interpreted Chaco great houses as earlier versions of the architecture seen in more recent Southwest societies. In particular, the Chaco houses appear strikingly similar to the large, well-known "apartment buildings" at Taos, New Mexico, in which many people have been living for centuries.A second theory contends that the Chaco structures were usedto store food supplies. One of the main crops of the Chaco people was grain maize, which could be stored for long periods of time without spoiling and could serve as a long-lasting supply of food. The supplies of maize had to be stored somewhere, and the size of the great houses would make them very suitable for the purpose.A third theory proposes that houses were used as ceremonial centers. Close to one house, called Pueblo Alto, archaeologists identified an enormous mound formed by a pile of old material. Excavations of the mound revealed deposits containing a surprisingly large number of broken pots. This finding has been interpreted as evidence that people gathered at Pueblo Alto for special ceremonies. At the ceremonies, they ate festive meals and then discarded the pots in which the meals had been prepared or served. Such ceremonies have been documented for other Native American cultures.V. TranslationDirections: Translate the following sentences into English, using the words given in the brackets.72. 想和我一起看电影的人请举手。

2017年上海市普陀区中考化学二模试卷(4月份)参考答案与试题解析一、选择题(共20分,每小题只有一个正确选项)1.(1分)下列气体会造成酸雨污染的是()A.一氧化碳B.二氧化碳C.二氧化硫D.氢气【解答】解:A.形成酸雨的主要气体是二氧化硫、二氧化氮,不是一氧化碳,故选项错误.B.形成酸雨的主要气体是二氧化硫、二氧化氮,不是CO?,故选项错课.C.形成酸雨的主要气体是二氧化硫、二氧化氮,故选项正确.D.形成酸雨的主要气体是二氧化硫、二氧化氮,不是氢气,故选项错误. 故选:C.2.(1分)下列自然现彖一定伴随化学变化的是()A、房屋倒塌B.冰雪融化C.火山喷发D.山体滑坡【解答】解:A、房屋倒塌过程中只是形状发生改变,没有新物质生成,属于物理变化.B、冰雪融化过程中只是状态发生改变,没有新物质生成,属于物理变化.C、火山喷发过程中有新物质生成,展于化学变化.D、山体滑坡过程屮只是形状发生改变,没有新物质生成,属于物理变化. 故选C.3.(1分)CFU中氢元素的化合价为()A.- 1B. +1C.・ 4 D・ +4【解答】解:碳元素显-4价,设氢元素的化合价是X,根据在化合物屮正负化合价代数和为零,可得:(-4) +4x=0,则x二+1价.故选:B.A.单质B.分子C.原子D.元素【解答】解:本题主要考查元素与微观粒子及物质的区别.宏观物质的组成,用宏观概念元素来表示;分子的构成,用微观粒子来表示.元素是具有相同核电荷数(即核内质子数)的一类原子的总称,是宏观概念,只讲种类,不讲个数.食品、药品、营养品、矿泉水等物质中的"碘、氟、钙、铁、锌"等不能以单质、分子、原子形式存在,而是指元素•通常用元素及其所占质量(质量分数)来描述•锌强化营养盐不是单质,故A错,分子原子是微观概念.故选D.5.(1分)下列反应和水的性质无关的是()A、氢气和氧气B.水和氧化钙C.水和二氧化碳D.无水硫酸铜和水【解答】解:A、氢气和氧气在点燃的条件下能够发生化学反应,和水的性质无关;B、水和氧化钙能够发生化学反应生成氢氧化钙,属于水的化学性质;C、水和二氧化碳能够发生化学反应生成碳酸,展于水的化学性质;D、无水硫酸铜和水能够发生化学反应生成五水硫酸铜,属于水的化学性质. 故选:A6.(1分)如果人体内的二氧化碳不能顺利排除体外,人体血液pH会()A.变大B.变小C.不变D.先变小后变大【解答】解:二氧化碳和水反应生成碳酸,生成的碳酸越多,则酸性越强pH值越小;所以如果人体中的C02不能顺利的排岀体外,人体的血液pH值将会变小. 故选B.7.(1分)某农作物因缺钾而生长不良,应给它施用的化肥是()A.K2SO4 B・(NHQ 2SO4 C・ Ca3(P04) 2 D. (NH2)2C0【解答】解:农作物因缺钾而生长不良,应给它施用含钾元素的化肥.故选A8.(1分)某氧化物不仅可作干燥剂,还能与酸反应,用于改良酸性土壤,该氧化物是()A.CaOB. CO2 C・出0 D. CuO【解答】解:A、氧化钙能与水反应,可以做干燥剂,属于金属氧化物,能与酸反应,与水反应生成的氢氧化钙显碱性,能用于改良酸性土壤,正确;B、二氧化碳不具有吸水性,不能用作干燥剂,错误;B、水不能用作干燥剂,错误;D、氧化铜不能吸水,不能做干燥剂,错误;故选A.9.(1分)对氧气的认识正确的是()A.氧气易溶于水,不能用排水法收集B.工业上利用物理变化得到氧气C.白磷在氧气燃烧,发出耀眼的白光D.空气中氧气浓度越高,越有利于生物生长【解答】解:A、氧气不易溶于水,为收集较纯净的氧气最好选择排水法收集.故错误;B、工业上利用液氧与液氮沸点不同,采取分离液态空气制取氧气,没有新物质生成,属于物理变化.故正确;C、白磷在氧气燃烧,产生大量白烟,放热,不是白光,故错误;D、空气中氧气浓度越高,呼吸作用旺盛,消耗的有机物多,释放的热量多,容易引起生物的腐烂,不一定有利于生物生长,故错误;答案:B10・(1分)如图是氮气的微观图,对于氮气认识错误的是()A.氮气是纯净物B.一个氮气分子由两个氮原子构成C.氮气化学式是N2D.氮气是由氮原子直接构成【解答】解:A.氮气是由--种物质组成的,属于纯净物,故正确;B.由分子结构模型可知,每个氮气分子是由两个氮原子构成的,故正确;C.由分子结构模型可知,每个氮气分子是由两个氮原子构成的,所以氮气的化学式为2,故正确;D.氮气是由氮分子构成的而不是由氮原子直接构成的,故错误.故选D.□・(1分)有关溶液说法止确的是()A.饱和溶液不能再溶解物质了B.溶液一定是混合物C.均一、稳定的液体一定是溶液D.不饱和溶液一定是稀溶液【解答】解:A、饱和溶液是指在一定温度下,一定量溶剂里不能再溶解某种溶质的溶液,叫做这种溶质的饱和溶液,可以溶解其它的溶质,故选项说法错误.B、溶液是均一、稳定的混合物,溶液的本质特征是均一性、稳定性,属于混合物,故选项说法正确.C、均一的、稳定的液体不一定是溶液,如水是均一的、稳定的液体,但不是混合物,不是溶液,故选项说法错误.D、浓稀溶液是溶液中所含溶质质量分数的大小,溶液是否饱和与溶液的浓稀没有必然联系,不饱和溶液可能是浓溶液,故选项说法错误.故选:B.12・(1分)硝酸钠在灼烧时产生的火焰焰色是()A.红色B.黄色C.青色D.紫色【解答】解:A、硝酸钠中含有钠元素,灼烧时火焰的颜色呈黄色,而不是红色,故选项错误.B、硝酸钠中含有钠元素,灼烧时火焰的颜色呈黄色,故选项正确.c、硝酸钠中含有钠元素,灼烧时火焰的颜色呈黄色,而不是青色,故选项错误. D、硝酸钠中含有钠元素,灼烧时火焰的颜色呈黄色,而不是紫色,故选项错误. 故选:B.23. (1分)除去下列物质中的少量杂质,选用的试剂正确的是()A. AB. B C・ C D・ D【解答】解:A、CaCb能与过量碳酸钠反应生成碳酸钙沉淀和氯化钠,能除去杂质但引入了新的杂质碳酸钠(过量的),不符合除杂原则,故选项所采取的方法错误.B、N aOH溶液能与稀盐酸反应牛成氯化钠和水,反而会把原物质除去,不符合除杂原则,故选项所采取的方法错误.C、N a2SO4能与适量的硝酸锁溶液反应生成硫酸顿沉淀和硝酸钠,能除去杂质但引入了新的杂质硝酸钠,不符合除杂原则,故选项所采取的方法错误.D、稀硫酸能与适量氯化顿溶液反应生成硫酸顿沉淀和盐酸,再过滤,能除去杂质且没有引入新的杂质,符合除杂原则,故选项所采取的方法正确.故选:D.14・(1分)取用固体药品的操作正确的是()【解答】解:A、取用固体药品氢氧化钠时,应用药匙取用,不能用手接触纯品, 图中所示操作错谋.B、向试管屮锌粒时,先将试管横放,用银子把锌粒放在试管口,再慢慢将试管竖立起来,图中所示操作错误.C、向试管中氯酸钾颗粒时,先将试管横放,用银子把氯酸钾颗粒放在试管口,再慢慢将试管竖立起来,图中所示操作错误.D、取用粉末状药品,试管横放,用药匙或纸槽把药品送到试管底部,图中所示操作正确.故选:D.15.(1分)当物质完全燃烧时,发生的燃烧反应与四种基本反应类型关系的说法中,正确的是()A.燃烧反应一定属于化合反应B.燃烧反应一定属于分解反应C.燃烧反应可能属于置换反应D.燃烧反应可能属于复分解反应【解答】解:A、石蜡燃烧生成二氧化碳和水,不屈于化合反应,故选项错误;B、燃烧反应都是可燃物与氧的反应,反应物有两种,不符合分解反应的定义,故选项错误;C、镁在二氧化碳中燃烧生成碳和氧化镁,符合置换反应的概念,故选项正确;D、复分解反应是两种化合物相互交换成分生成另外两种化合物的反应,故燃烧反应不可能属于复分解反应,故选项错课;故选C16.(1分)下列应用一定与中和反应原理无关的是()A.施用熟石灰改良酸性土壤B.服用含Al (0H) 3的药物治疗胃酸过多C.用熟石灰和硫酸铜配制波尔多液D.用NaOH溶液洗涤石油产品中的残余硫酸【解答】解:A、熟石灰是氢氧化钙的俗称,与酸性土壤中的酸反应牛成盐和水,展于中和反应.B、Al (OH) s与胃液屮的盐酸反应生成氯化铝和水,是酸与碱的反应,屈于中和反应.C、熟石灰与硫酸铜溶液反应生成氢氧化铜沉淀和硫酸钙,不是酸与碱的反应,不属于中和反应.D、N aOH溶液与硫酸反应生成硫酸钠和水,是酸与碱的反应,属于中和反应. 故选C ・17.(1分)如图所示,两个甲分子反应生成X个乙分子和Y个丙分子,则从图示获得的信息中,正确的是()•和。

2017上海各区二模化学习题答案详解6一、2017年上海市嘉定区二模卷答案详解44 Na2OH+H2SO4==Na2SO4+H2OA 对Na2OH+CuSO4== Na2SO4+Cu(OH)2↓B 错误BaCl2+ H2SO4==BaSO4↓+2HCl, BaCl2+ Na2SO4== BaSO4↓+2NaCl H2SO4和Na2SO4都能与BaCl2反应生成白色沉淀C 对氢前金属与酸反应生成氢气D 对酚酞遇碱变红45 氢氧化钠变质的方程式2NaOH+CO2==Na2CO3+H2O1)对Na2CO3+2HCl==2NaCl+CO2↑,CO2+Ca(OH)2==CaCO3↓+H2O 能检验是否变质2)错Na2CO3+Ca(OH)2== CaCO3↓+2NaOH, 滤液中存在反应后生成的NaOH,加酚酞只能显示滤液呈碱性,不能说明样品里是否含有NaOH,有可能存在完全变质的情况,而这种方法不能确定成分。
3)错Na2CO3+2HCl==2NaCl+CO2↑+H2O,NaOH+HCl==NaCl+H2O 因为氢氧化钠固体能吸收水和CO2气体,导致反应后的CO2质量测量不准确,无法测定纯度。
4)对,Na2CO3+Ca(OH)2== CaCO3↓+2NaOH,反应后得到NaOH,故滤掉固体得到纯净的NaOH。
答案 D(14)46 A 错,锌与足量盐酸反应完之后质量应该为零,图中不为0B 错误,Cu+2AgNO3==Cu(NO3)2+2Ag, 1mol的铜质量为63.5克,生成2mol的银质量为216克,因此固体质量是逐渐增加的,与图相反。
C正确Zn+2HCl==ZnCl2+H2↑Mg+2HCl==MgCl2+H2↑2mol稀盐酸,需要24克镁,65克锌,镁的活性强,产生氢气的速度更快。
D 错误,2KClO3=MnO2=2KCl+3O2↑反应时氯酸钾不断消耗,固体质量不断减少,而锰元素的质量不变,所以质量分数反而慢慢变大。

上海市普陀区2017届高三上学期质量调研化学试题考生注意:1、本卷满分150分,考试时间120分钟。
2、本考试设试卷和答题纸两部分,试卷包括试题与答题要求;所有答案必须涂或写在答题纸上;做在试卷上一律不得分。
3、答题前,考生务必在答题纸上用钢笔或圆珠笔在答题纸正面清楚地填写姓名、准考证号,并将核对后的条形码贴在指定位置上。
4、答题纸与试卷在试题编号上是一一对应的,答题时应特别注意,不能错位。
相对原子质量:H—1 C—12 N—14 O—16 Cl-35.5 S—32Na—23 Al—27 Fe—56 Cu—64一、选择题(本题共10分,每小题2分,每题只有一个正确选项)1、关于汽油、甘油和花生油的叙述正确的是A.都属于油脂 B.都无固定的沸点C.都能浮在水面上 D.只有花生油能与氢氧化钠溶液反应2、在非室温条件下可以使用的仪器是A.漏斗B.量筒C.容量瓶D.滴定管3、按照有机物的命名规则,下列命名正确的是A .CH 2Br -CH 2Br 二溴乙烷B .CH 3OOCCH 3 甲酸乙酯C .CH C 17H 35COO 2CH 2C 17H 35COO C 17H 35COO 硬脂酸甘油脂 D .CCH CH 3CH 3CH 3CH 3OH3,3-二甲基-2-丁醇4、关于碘及其化合物的说法正确的是A .“碘遇淀粉变蓝色”,“碘”指碘元素B .为了补碘,要多食用含高碘酸的食物C .I - 与IO 3- 可以大量共存于溶液中D .通过“取样→灼烧→溶解→过滤→萃取”可从海带中提取单质碘5、铝的某种超原子结构(Al 13)具有40个价电子时最稳定。
请预测稳定的Al 13所带的电荷数为A .-1B . +2C .0D . +3二、选择题(本题共36分,每小题3分,每题只有一个正确选项) 6、通常情况下,前者无法决定后者的是A .原子的核外电子排布----元素的金属性强弱B .化合物的内在结构----电解质的相对强弱C .反应温度的高低----化学平衡的移动程度D .反应物的化学性质----化学反应速率的快慢7、实验装置是为达成实验目的选用的。

上海市普陀区2015届高三化学第二次模拟考试试题(扫描版)普陀区2014学年第二学期高三质量调研化学试卷参考答案及评分标准2015.4一、选择题(本题共10分,每小题2分,只有一个正确选项)1 2 3 4 5D C D B B6 7 8 9 10 11 12 13 14 15 16 17A A CB B D BC BD C C三、选择题(本题共20分,每小题4分。
只有一个正确选项的,多选不给分;有两个正18 19 20 21 22AC A AC BD AB四、(本题共12分)23、2:3;0.6NA (1分×2)24、溶液变蓝;(1分)5I- + IO3 - +6 CH3COOH →3I2 + 3H2O + 6CH3COO-(2分)25、c(H+) c(ClO-)/c(HClO) ;(1分)10-7.5 或3.16×10-8(1分)26、大于(2分)27、差;(1分) 温度升高,(溶解)平衡①逆向移动,Cl2(aq)浓度减少,使得(化学平衡)②逆向移动,c(HClO) 减少,(杀菌效果变差。
) (2分)五、(本题共12分)28、; (2分)29、C (1分)、 Na2SiO3 + CO2 + H2O→Na2CO3+H2SiO3↓ 合理即可。
(1分)30、ac (2分)31、(1)ad (2分);(2)(0.16/t)mol/(L.min)(2分); 0.32(1分)32、正(1分)六、(本题共12分)33.缺少温度计;大试管接触烧杯底部(或其他合理说法)。
(2分)34.使苯与混酸混合均匀;能及时移走反应产生的热量(或其他合理答案)。
(2分)35.浅黄色;硝基苯、苯(2分)。
b 、d (2分)36.②氢氧化钠溶液;分液漏斗 ③水洗、分离或水洗 ⑤蒸馏。
(2分)37.(1)温度过高引起硝酸的分解、苯的挥发。
(2)温度过高,有其他能溶于硝基苯的产物生成,或“有副反应,生成其他产物”等合理答案。


17年高三二模大题汇编——有机部分一、(17年长宁、金山、宝山二模)本题共15分:请回答下列问题:30. A 的名称是__________________,含有的官能团名称是__________________________,实验室由A 转化为乙烯的反应条件为______________________________。
31. B 和A 反应生成C 的化学方程式为__________________________________,该反应的类型为______________________________________。
32. F 的结构简式为_____________________。
33. 写出D 的同分异构体的结构简式______________________。
34. 乙炔也是常见的一种化工原料,它可以制取很多化工产品,例如:聚氯乙烯塑料。
写出由乙炔合成聚氯乙烯的合成路线。
(合成路线常用的表示方式为:A B −−−−→−−−−→反应试剂反应试剂反应条件反应条件……目标产物)CH 2CH 2有机物F是合成多种药物的重要中间体,一种合成路线如下:回答下列问题:38.写出反应类型。
反应①是_____________反应,反应④是______________反应。
39.化合物A中有___________种官能团,写出含氧官能团的结构式________________________ 。
40.化合物C的俗名是肉桂醛,其分子式是________________________。
写出肉桂醛跟新制Cu(OH)2在煮沸条件下发生反应的化学方程式__________________________________________ 。
41.由肉桂醛()和乙酸制备的合成路线如下:________________________________________________________________________请将该合成路线补充完整(无机试剂任选)。
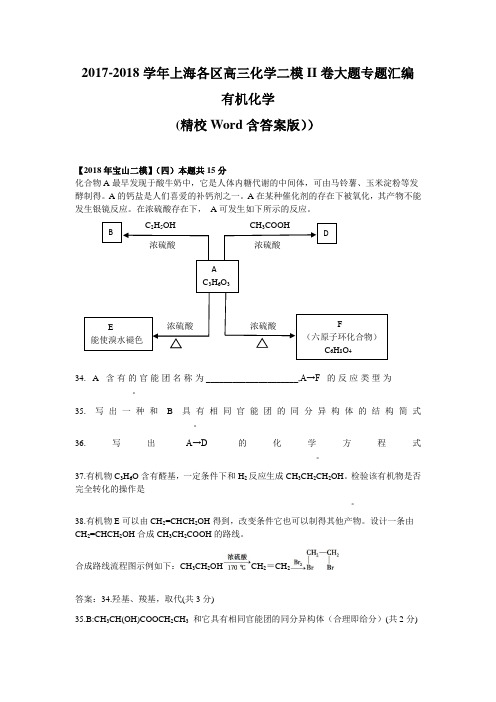
B DAC3H6O3E能使溴水褪色F(六原子环化合物)C6H8O42017-2018学年上海各区高三化学二模II卷大题专题汇编有机化学(精校Word含答案版))【2018年宝山二模】(四)本题共15分化合物A最早发现于酸牛奶中,它是人体内糖代谢的中间体,可由马铃薯、玉米淀粉等发酵制得。
A的钙盐是人们喜爱的补钙剂之一。
A在某种催化剂的存在下被氧化,其产物不能发生银镜反应。
在浓硫酸存在下,A可发生如下所示的反应。
C2H5OH CH3COOH浓硫酸浓硫酸浓硫酸浓硫酸34. A含有的官能团名称为_____________________,A→F的反应类型为____-_____________。
35. 写出一种和B具有相同官能团的同分异构体的结构简式___________________________。
36.写出A→D的化学方程式_______________________________________________________。
37.有机物C3H6O含有醛基,一定条件下和H2反应生成CH3CH2CH2OH。
检验该有机物是否完全转化的操作是_______________________________________________________________。
38.有机物E可以由CH2=CHCH2OH得到,改变条件它也可以制得其他产物。
设计一条由CH2=CHCH2OH合成CH3CH2COOH的路线。
合成路线流程图示例如下:CH3CH2OH CH2=CH2答案:34.羟基、羧基,取代(共3分)35.B:CH3CH(OH)COOCH2CH3和它具有相同官能团的同分异构体(合理即给分)(共2分)浓硫酸36. CH3CH(OH)COOH+CH3COOH →CH3CH(COOH)OOCCH3+H2O (共3分)37.取样,加入新制氢氧化铜,煮沸,观察是否有砖红色沉淀生成,若有砖红色沉淀生成,则未全部转化;若没有砖红色沉淀生成,则已全部转化。

化学试卷 第1页(共8页) 化学试卷 第2页(共8页)绝密★启用前2017年上海市普通高中学业水平等级性考试化 学满分100分,考试时间90分钟。
可能用到的相对原子质量:H 1— C 12— O 16— S 32— Cl 35.5— Na 23—Mg 24— Fe 56—一、选择题(本题包括25小题,每小题2分,共50分,每小题只有一个选项符合题意) 1.世界气候大会与2009年12月在丹麦首都哥本哈根召开,商讨2012至2020年全球温室气体减排协议。
下列物质属于温室气体的是( )A .2NB .2HC .2COD .2O 2.海洋是一个巨大的宝藏,期待着人们的开发和利用。
下列物质不经过化学变化就能从海水中获得的是( ) A .单质溴 B .单质镁 C .烧碱D .食盐3.下列行为不是..健康文明的生活方式的是( )A .不偏食,注意营养均衡B .每天坚持适度锻炼C .沉迷网络聊天、游戏D .不吸烟、不酗酒,远离毒品4.垃圾处理无害化、减量化和资源化逐渐被人们所认识。
垃圾的分类收集是实现上述理念的重要措施。
某垃圾箱上贴有如下图所示的标志,向此垃圾箱中丢弃的垃圾是( )A .危险垃圾B .可回收物C .有害垃圾D .其他垃圾5.当光束通过下列分散系时,能观察到丁达尔效应的是( )A .盐酸B .()3Fe OH 胶体C .氯化钠溶液D .4CuSO 溶液6.实验室制取下列气体时,不能用排气法收集,而只能用排水法收集的是( ) A .3NHB .2ClC .2COD .NO 7.实验室中,有关试剂的保存方法错误的是( )A .烧碱溶液保存在带玻璃塞的试剂瓶中B .液溴用水封保存C .少量金属钠保存在煤油中D .浓硝酸保存在棕色细口瓶中 8.下列实验操作或装置正确的是( )点燃酒精灯蒸馏 过滤 稀释浓硫酸ABC D9.据报载我国最近合成新的同位素,其中一种是18572Hf (铪),它的质子数是( ) A .72 B .113C .185D .257 10.下列物质中,只含..共价键的是( )A .NaClB .2Na OC .HClD .NaOH11.23Na CO 俗名纯碱,下面是对纯碱采用不同分类法的分类。

2017年高三二模汇编-选择部分有机选择1. (17年浦东新区二模)实验室制备乙酸乙酯和乙酸丁酯采用的相同措施是()A .水浴加热B .冷凝回流C .用浓硫酸做脱水剂和催化剂D .乙酸过量【难度】★ 【答案】C2. (17年长宁、金山二模)6.下列化工生产涉及的变化主要是物理变化的是 ()A .煤的干馏B .石油的分馏C .石油的裂化D .石油的裂解【难度】★ 【答案】B3. (17年长宁、金山二模)7.按照有机物的命名规则,下列命名正确的是 ()A .1,2-二甲基戊烷B .2-乙基丁烷C .3-乙基己烷D .3,4-二甲基戊烷【难度】★ 【答案】C4. (17年长宁、金山、青浦、宝山二模)14.布洛芬片常用来减轻感冒症状,其结构简式如图,下列有关说法错误的是()A .布洛芬的分子式为C 13H 18O 2B .布洛芬与苯乙酸是同系物C .1mol 布洛芬最多能与3mol 氢气发生加成反应D .布洛芬在苯环上发生取代反应,其一氯代物有4种 【难度】★★ 【答案】D5. (17年杨浦二模)6.烷烃命名中常使用三套数字,甲、乙、丙……,1、2、3……,一、二、三……。
其中“一、二、三……”是说明 ( )A .碳原子数B .烷基位置编号C .氢原子数D .同种烷基数目【难度】★ 【答案】D6. (17年杨浦二模)16.在加热条件下,乙醇转化为有机物R 的过程如图所示,其中错误的是()A .R 的化学式为C 2H 4OCH CH 3COOHCH 2CH CH 3CH3B.乙醇发生了还原反应C.反应过程中固体有红黑交替变化的现象D.乙二醇(HO-CH2-CH2-OH)也能发生类似反应【难度】★★【答案】B7.(17年徐汇二模)2.我国油气资源匮乏而煤储量相对丰富。
将煤转化为水煤气(CO、H2),不可能产生的效果是()A.得到相对清洁的能源B.提高了能源利用率C.增加了单位质量煤的热值D.便于管道输送【难度】★【答案】C8.(17年徐汇二模)3.以下化石能源的加工方式能大量得到乙烯的是()A.石油裂解B.石油裂C.石油分馏D.煤焦油分馏【难度】★【答案】A9.(17年徐汇二模)5.右图是一种有机物的模型,该模型代表的有机物可能是()A.饱和一元醇B.羟基酸C.羧酸酯D.饱和一元醛【难度】★【答案】B10.(17年徐汇二模)9.黄酒酿造过程中有部分乙醇最终转化为具有芳香气味的物质,该过程中发生的反应有()A.还原反应B.氧化反应C.加成反应D.聚合反应【难度】★【答案】B11.(17年松江二模)乙醇转化为乙醛,发生的反应为()A.取代反应B.加成反应C.消除反应D.氧化反应【难度】★【答案】D12.(17年松江二模)催化加氢可生成3-甲基戊烷的是()【难度】★【答案】C13.(17年松江二模)莽草酸结构简式如图,有关说法正确的是()A.分子中含有2种官能团B.1mol莽草酸与Na反应最多生成4mol氢气C.可与乙醇、乙酸反应,且反应类型相同D.可使溴的四氯化碳溶液、酸性高锰酸钾溶液褪色,且原理相同【难度】★★【答案】C14.(17年青浦二模)8.按照有机物的命名规则,下列命名正确的是()A.1,2-二甲基戊烷B.2-乙基丁烷C.3-乙基己烷D.3,4-二甲基戊烷【难度】★【答案】C15.(17年普陀二模)分子式为C n H2n+1Cl(n>1)的卤代烃不能发生消去反应,n的最小值是()A.3 B.4 C.5 D.6【难度】★【答案】C16.(17年普陀二模)14.山梨酸(CH3-CH=CH-CH=CH-COOH)是一种高效安全的防腐保鲜剂。

2017上海各区二模化学习题答案详解1一、2017年上海市闵行区二模卷答案详解41 A错,锌和铁都是氢前金属,都能与酸反应产生氢气,无法比较2者活动性强弱。
应该用一种金属与含另一种金属的盐的置换反应来证明。
B 错,H2O2溶液是浓度不同,不能比,变量只能有1个C 错,溶质、溶剂都不同,不能比D 正确,浓盐酸有挥发性,能使石蕊试剂变红42 NaOH变质CO2+NaOH==Na2CO3+H2OA 正确,CO2不能Na2CO3反应,无法证明B 错Na2CO3+Ca(OH)2==CaCO3↓+2NaOH,有白色沉淀C 错Na2CO3+BaCl2==BaCO3↓+NaCl,有白色沉淀D 错CaCO3+2HCl==CaCl2+H2O+CO2↑有气泡产生44 除去杂质的要点:⑴选择的试剂不能与需要提纯的物质反应⑵试剂与杂质反应的产物可以是需要提纯的物质,也可以是新物质(必须是↑↓H2O),不能是可溶性的新物质A错,铁丝在含氧量太少的氮气中无法燃烧,不能除去O2B错,H2O+CaO==Ca(OH)2,选择的试剂与需要提纯的物质反应了C正确,Cu(NO3)2不与Cu反应,Cu+2AgNO3== Cu (NO3)2+2Ag,铜能置换硝酸银中的银,生成物恰好是Cu (NO3)2D错,KNO3不能与CuSO4反应(无↑↓H2O产生),CuSO4+2KOH ==K2SO4+Cu(OH)2↓Cu(OH)2↓可过滤,但引入了新的杂质K2SO445 CaCO3=煅烧= CaO+CO2↑1 1 1100g/100g/mol=1mol m Cao=1*(40+16)=56g<78g所以有部分CaCO3未完全分解成CaO,CO2,这部分CaCO3与稀盐酸反应生成了CO2 CaCO3=煅烧= CaO+CO2↑CaCO3+2HCl==CaCl2+H2O+CO2↑X X Y Y设Xmol的CaCO3分解,生成Xmol CO2;Y mol的CaCO3与酸反应生成Ymol CO2 X+Y=1mol 所以共生成1 mol CO2 答案:DA 正确,Zn (NO3)2,Cu (NO3)2不与稀盐酸反应,NaCl+AgNO3==AgCl↓+NaNO3,所以是上表5的情况,固体是AgB 正确,溶液甲是蓝色,说明含有,Cu (NO3)2,上表345都符合,一定有Ag,3有CuC正确,固体乙有Ag Cu Zn,是上表1,溶液是Zn (NO3)2D错误,氢前金属与稀盐酸反应生成氢气,说明固体乙一定含Zn,符合上表1情况,此时溶液甲中只有Zn (NO3)2,不符合47①根据物质分类,甲橱放非金属单质,所以放甲橱。
一、选择题(本题共40分,每小题2分。
每题只有一个正确选项)1.不能用单质直接反应得到的是A.NaClB. MgCl2C.AlC13D.FeC122.关于氮肥的说确的是A.尿素属于铵态氮肥 B.植物吸收氮肥属于氮的固定C.使用碳按应深施盖土 D.硫铵与石灰混用肥效增强3.可用铝热反应冶炼的金属是A.WB. NaC.MgD. Hg4.可用浓硫酸干燥的气体是A.SO2B. H2SC. HBrD. NH35.只表示一种微粒的化学用语是A. B.X:X C.ns l D. X-X6.为了检验某固体物质中是否含有NH4+,一定用不到的试剂或试纸是A. NaOH溶液 B.浓盐酸 C.稀硫酸 D.红色石蕊试纸7.可检验FeC13溶液中是否含FeCl2的试剂是A. KSCN溶液 B.氯水 C. NaOH溶液 D.酸性KMnO4溶液8.分子式为C n H2n+l Cl(n>l)的卤代烃不能发生消去反应,n 的最小值是A.3B.4C.5D.69.能证明NaCl是离子化合物的事实是A.常温下是固体 B.易溶于水 C.水溶液能导电 D.熔融态能导电10.短周期元素W、Q、X、Y在元素周期表中的位置如右图,其中X是两性金属元素。
则A.简单离子半径:X<Q B.气态氢化物的稳定性:Y>QC.X的族序数大于周期数D. Y氧化物对应水化物是强酸11.有关漂粉精的认识错误的是A.漂粉精的主要成分是次氯酸钙 B.漂粉精露置在空气中会失效C.漂粉精水溶液具有漂白性 D.漂粉精杀菌效果随pH降低而增强12.下列图像与选项对应的是13.为了除去物质中的杂质(括号为杂质),选用试剂正确的是物质选用试剂A NaBr溶液(NaI) 溴水B CuCl2溶液(FeCl3) 铜粉C 乙烷(乙烯)氢气D 溴苯(苯)液溴14.山梨酸(CH3-CH=CH-CH=CH-COOH)是一种高效安全的防腐保鲜剂。
有关山梨酸的说确的是A.属于二烯烃 B.和Br2加成,可能生成4种物质C.1mol可以和3molH2反应D.和CH3CH218OH反应,生成水的摩尔质量为20g/mol 15.正确认识铁制品的电化腐蚀与防护方法的是A.铁制品腐蚀时既可做正极也可做负极B.铁制品腐蚀时铁电极发生反应为:Fe-3e→Fe3+C.铁制品腐蚀时或发生析氢腐蚀或发生吸氧腐蚀D.铁制品连接电源正极可防止腐蚀16.下列各项应用涉及的化学原理完全相同的是A.用氯化铁或液氯处理废水 B.用铁槽车或铝槽车运输浓硫酸C.用二氧化硫或氯气漂白织物 D.用亚硫酸钠或氨水处理二氧化硫尾气17.向0.lmol/LCH3COOH溶液中加入少量NaOH固体(忽略温度、体积变化),增大的是A.氢离子浓度 B.水的电离程度 C.水的离子积 D.醋酸浓度18.对化工生产认识正确的是A.海水提溴:一般需要经过浓缩、氧化、提取三个步骤B.合成氯化氢:通入H2的量略大于C12 ,可以使平衡正移C.合成氨:采用500℃的高温,有利于增大反应正向进行的程度D.侯德榜制碱法:析出NaHCO3 的母液中加入消石灰,可以循环利用NH319.向含有下列离子的各组溶液中分别通入足量相应气体,各离子还能大量存在的是A.Fe3+、SO42-,通氨气 B.Na+、HCO3-,通氯气C. Fe2+、NO3-,通氯化氢D.Ca2+、Br-,通二氧化硫20. 25℃时,将某强酸和强碱溶液按10:l的体积比混合后溶液恰好呈中性,则混合前强酸和强碱溶液的pH之和为A.9B.11C.13D.15二、综合题(本题共60分)(一)本题共15分工业上可用微生物处理含KCN的废水。
第一步是微生物在氧气充足的条件下,将KCN 转化成KHCO3和NH3(最佳pH:6.7~7.2);第二步是把氨转化为硝酸:NH3+2O2HNO3+H2O。
请完成下列填空:21.写出第一步反应的化学反应方程式___________,第二步反应的还原产物是_____(填写化学式)。
22.在KCN中,属于短周期且原子半径最大的元素是____,氮原子最外层电子的运动状态有___种。
水的电子式是_______。
23.比较碳和氮元素非金属性强弱,化学反应方程式为_____________。
24.室温下,0.lmol/LK2CO3、KCN、KHCO3溶液均呈碱性且pH依次减小,在含等物质的量的KCN、KHCO3混合溶液中,阴离子(除OH-)浓度由大到小的顺序是________。
25.工业上还常用抓氧化法处理含KCN的废水:KCN+2KOH+Cl2→KOCN+2KCl+H2O,2KOCN+4KOH+3C12→N2+6KCl+2CO2+2H2O。
两者相比,微生物处理法的优点与缺点是《各写一条)。
优点:______________________,缺点:________________。
(二)本题共15分度气中的H2S通过高温热分解可制取氮气:2H2S(g)2H2(g)+ H2S(s)。
现在3L密闭容器中,控制不同温度进行H2S分解实验。
26.某温度时,测得反应体系中有气体1.3lmol,反应tmin后,测得气体为l.37mol,则tmin H2的生成速率为_____。
27.某温度时,H2S的转化率达到最大值的依据是_____(选填编号)。
a.气体的压强不发生变化 b.气体的密度不发生变化c.不发生变化 d.单位时间里分解的H2S和生成的H2一样多28.实验结果如右图。
图中曲线a表示H2S的平衡转化率与温度关系,曲线b表示不同温度下、反应经过相同时间且未达到化学平衡时H2S的转化率。
该反应为_____反应(填“放热”或“吸热”)。
曲线b随沮度的升高,向曲线a通近的原因是______________。
在容器体积不变的情况下,如果要提高H2的体积分数,可采取的一种措施是___________。
29.使1LH2S与20L空气(空气中O2体积分数为0.2)完全反应后恢复到室温,混合气体的体积是______L 。
若2gH2S完全嫩烧后生成二氧化硫和水蒸气,同时放出29.4kJ的热量,该反应的热化学方程式是_____________。
(三)本题共15分实验室中模拟纯碱工业制法,进行了如下三步实验:①将CO2、NH3通入H2O中制得NH4HCO3,② NH4HCO3和饱和食盐水反应生成小打,③将小打制成纯碱。
30.步骤①先通入水中的气体是______。
析出NH4HCO3的反应有两步,第二步反应的离子方程式为_____________。
31.步骤②生成了含少量NaCl的小打,现用滴定法测定其中NaHCO3的含量。
过程为:称取一定质量样品,配成25OmL溶液,取出25.00mL用0.l000mol/L的盐酸滴定。
实验中所需的定量仪器除滴定管外,还有_____、_____。
所用的指示剂是__________。
32.步骤③生成了含少量NaHCO3的纯碱。
若用重量法(限用试剂:稀盐酸)测定其中Na2CO3含量,请设计实验方案:_____________________。
若该样品中Na2CO3含量的准确值为99.5%,而上述实验结果为97.6%,则测定的相对误差为_____,可能造成该实验误差的原因是_________________。
(四)本题共15分维生素类药物中间体。
(),可按如下线路合成:其中A-G分别代表一种有机化合物,合成路线中的部分产物及反应条件已略去。
己知:完成下列填空:33. A的名称是______,F中的官能团____________。
34.写反应类型:反应①________,反应②___________。
35. G可以转化为化合物M(C6H10O2),写出满足下列条件的2种M的结构简式:_____、______。
①分子中除羟基外还有一种碳碳不饱和键;②链状且不存在结构;③分子中只有3 种不同化学环境的氢原子。
36.设计一条由A制备B的合成路线。
(合成路线常用的表示方式为:)一、选择题(本题共40分)每小题2分,选对得2分,选错得0分。
1-10:DCAAB CDCDA 11-20:ABABC BBADD二、综合题(本题共60分)21.2KCN+O 2+4H 2O 2KHCO 3+2NH 3;HNO 3、H 2O ;22.碳,5,;23.NaHCO 3+HNO 3→NaNO 3+CO 2+H 2O ;24.c(HCO 3-)>c(CN -)>c(CO 32-);25.优点:不存在液氯泄露。
缺点:微生物适应性差。
26.min)/(t04.0•L mol ; 27.a 、c ;28.吸热;随温度升高,反应速率加快,达到平衡所需要的时间变短;升高反应温度,及时分离S2气体等。
29.19.5,2H 2S(g)+3O 2(g)→2SO 2(g)+2H 2O(g)+999.6kJ(三)本题共15分30.氨气,CO 2+2NH 4++CO 32-+H 2O→2NH 4HCO 3↓31.电子天平、250mL 容量瓶;甲基橙;32.称取一定质量的该样品,加入足量稀盐酸,充分反应后,加热蒸干至恒重,称量所得固体氯化钠的质量;-1.9%;蒸发过程中有少量晶体溅出。
(四)本题共15分33.2-甲基丙烯,羧基、羟基;34.加成反应,取代反应;35. ;;;(任写两个) 36.(6分)。