Raman spectroscopic study on pressure-induced amorphization in nanocrystalline anatase (TiO2)
- 格式:pdf
- 大小:69.43 KB
- 文档页数:4
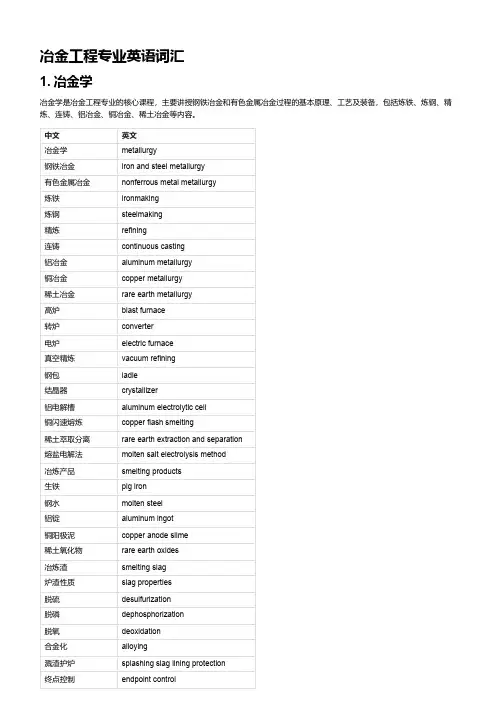
冶金工程专业英语词汇1. 冶金学冶金学是冶金工程专业的核心课程,主要讲授钢铁冶金和有色金属冶金过程的基本原理、工艺及装备,包括炼铁、炼钢、精炼、连铸、铝冶金、铜冶金、稀土冶金等内容。
中文英文冶金学metallurgy钢铁冶金iron and steel metallurgy有色金属冶金nonferrous metal metallurgy炼铁ironmaking炼钢steelmaking精炼refining连铸continuous casting铝冶金aluminum metallurgy铜冶金copper metallurgy稀土冶金rare earth metallurgy高炉blast furnace转炉converter电炉electric furnace真空精炼vacuum refining钢包ladle结晶器crystallizer铝电解槽aluminum electrolytic cell铜闪速熔炼copper flash smelting稀土萃取分离rare earth extraction and separation熔盐电解法molten salt electrolysis method冶炼产品smelting products生铁pig iron钢水molten steel铝锭aluminum ingot铜阳极泥copper anode slime稀土氧化物rare earth oxides冶炼渣smelting slag炉渣性质slag properties脱硫desulfurization脱磷dephosphorization脱氧deoxidation合金化alloying溅渣护炉splashing slag lining protection终点控制endpoint control中文英文出钢操作tapping operation凝固传热机制solidification heat transfer mechanism凝固结构与缺陷solidification structure and defects氧化还原反应oxidation-reduction reaction造渣反应与造渣制度slagging reaction and slagging system2. 冶金物理化学冶金物理化学是冶金工程专业的基础理论课程,主要讲授冶金过程中涉及的物理化学原理和方法,包括平衡与相图、溶液理论、电化学、表面与胶体化学、传递现象等内容。

第三代半导体材料氮化镓的拉曼光谱分析作者:***来源:《无线互联科技》2024年第03期摘要:第三代半导体材料中氮化镓是高频电子器件、大功率电子器件和微波功率器件制造领域的首选材料。
为了实现高质量氮化镓材料的外延生长,并且精准表征氮化镓外延材料的特性,文章对氮化镓外延材料进行了深入的拉曼光谱分析。
实验结果表明,对氮化镓外延材料进行拉曼光谱分析时最佳扫描范围是100~1 000 cm-1、最佳曝光时间是5 s、最佳光孔直径为100 μm,从而更精准地表征氮化镓外延材料,进而对微波功率器件的性能提升起到推动作用。
关键词:第三代半导体材料;氮化镓;拉曼光谱中图分类号:TN304 文献标志码:A0 引言第三代半导体材料出现后,逐步形成以氮化镓材料[1]为代表的一系列半导体材料,其中还包括碳化硅和金刚石等。
第三代半导体材料有其独有的特性,比如禁带宽度大、电子迁移率高以及击穿场强大等[2]。
在半导体材料进行异质外延时,有2种因素会导致外延层产生应变。
拉曼光谱测试仪就是利用这一原理进行工作。
这2种因素包括:衬底材料的膨胀系数与外延层的膨胀系数存在较大差异、衬底材料的晶格常数和外延材料的晶格常数存在较大差异。
在半导体中引入残余应力,会使得半导体能带结构以及外延层的结构性质产生变化,当应力较大时还会引起外延层产生裂纹。
拉曼峰的位置能够显示样品的成分分布,其中包括化学组成、结构和形态等。
峰位位移能够显示样品的属性分布,其中包括应力和温度。
拉曼散射光谱在研究材料各项性能和晶格等方面起到很大作用,其优势在于非接触性、非破坏性,并且不使用特殊的样品制备[3-4]。
氮化物半导体中存在特殊的化学键,这种化学键属于共价键和离子键的混合型,并且很容易受激光辐射,正因如此更适合用拉曼散射来进行分析[5]。
若要提升微波功率器件的性能,需要从提高第三代半导体材料氮化镓的晶体质量出发,对氮化镓材料进行深入详尽的拉曼光谱分析。
1 实验方法氮化镓外延材料中产生残余热应变,这是由衬底材料的膨脹系数与氮化镓外延层的膨胀系数存在巨大差异造成的。

白云石I相到Ⅱ相高压相变行为的拉曼光谱研究作者:张璐牛菁菁翟天雷秦善来源:《当代化工》2019年第11期Raman Spectroscopic Study on the High-pressure PhaseTransition Behavior of Dolomite;From Phase I to Phase IIZHANG Lu, NIU Jing-jing, ZHAI Tian-lei,;QIN;Shan(Key Laboratory of Orogenic Belts and Crustal Evolution, MOE, School of Earth and Space Sciences, Peking University,Beijing 100871, China)作为地壳中分布最广的含碳物质之一,碳酸盐广泛分布于地壳,并且对碳循环的演化过程具有重要作用。
碳循环是指深海沉积形成的碳酸盐受洋壳俯冲作用而向下运移进入地球深部,在深部通过变质和火山作用再经地质作用回到地表的过程[1]。
在此过程中,压力、温度和氧逸度共同作用使得碳酸盐在进入地球深部时发生分解、相变等一系列变化,其中的含碳物质也随之赋存[2,3]。
因此研究碳酸鹽矿物在地球深部的晶体结构和性质对理解深部碳循环具有重要的意义。
进入地球深部的碳酸盐矿物主要有白云石CaCO3、方解石MgCO3和菱镁矿CaMg(CO3)等。
作为碳酸盐中最重要的矿物之一,它们在高温高压下的稳定性和结构变化也受到了学者2的关注。
在地幔顶部对应的温压条件下白云石可以分解成文石和菱镁矿[4],而菱镁矿因其较强的结构稳定性被看作是深部碳的潜在载体[5,6];方解石在高压下会发生一系列相变[7-9]。
白云石矿物的分子式为CaMg(CO3)2,为三方晶系晶体,菱面体结构,空间群R`3,Z=3。
白云石的晶体结构见表1和图1。
其中Ca2+八面体和Mg2+八面体沿三次轴相互交替排列,使得C-O键面外弯曲振动相对于方解石族矿物的拉曼位移发生裂变,形成面外弯曲振动和反面外弯曲振动,分别位于292;cm-1和331 cm-1,而其它拉曼峰均无裂变[10]。

6Who should attendFrom Monday 9 am to Wednesday 5:30 pmDates: February 11-13, 2019 May 13-15, 2019 June 24-26, 2019 October 7-9, 2019November 18-20, 2019Users of HORIBA Scientific Raman spectrometers • A cquire theoretical and practical knowledge on Raman spectrometers • L earn how to use the software • L earn methodology for method development and major analytical parameters • H ow to set up an analytical strategy with an unknown sample • H ow to interpret results• L earn how to follow the performances of theRaman spectrometer over the time.Day 1• The theory of the Raman principle • R aman Instrumentation • P ractical session – System and software presentation, Acquisition Parameters: - L abSpec 6 presentation and environment: useraccounts, file handling, display of data, basic functions - S et up of acquisition parameters and singlespectra measurement - Templates & ReportsDay 2• Analysis of Raman spectra • P ractical session: Raman spectrum measurement and Database Search - O ptimization of the parameters: how to chosethe laser, the grating, the confocal hole, the laser power- How to use the polarization options - Library Search using KnowItAll software - How to create databasesRaman imaging • H ow to make a Raman image (1D, 2D and 3D) • D ata evaluation: cursors, CLS fitting, peakfitting•Image rendering, 3D datasets •Fast mapping using SWIFT XSDay 3Data processing• Processing on single spectra and datasets • Baseline correction • Smoothing • Normalization• Spectra subtraction, averaging • Data reduction • Methods• Practical exercisesCustomer samples: Bring your own samples!Duration: 3 daysReference: RAM1Raman Microscopy for Beginners7Acquire technical skills on DuoScan, Ultra Low Frequency (ULF), Particle Finder or TERS.Users of HORIBA Scientific Raman spectrometers who already understand the fundamentals of Raman spectroscopy and know how to use HORIBA Raman system and LabSpec Software. It is advised to participate in the basic Raman training first (RAM1).Introduction to DuoScan• Principle and hardwareDuoScan Macrospot• Practical examplesDuoScan MacroMapping• Practical examplesDuoScan Stepping Mode• Practical examplesCustomer samples: Bring your own samples!Presentation of the ULF kit• Principle and requirements • Application examplesInstallation of the ULF kitIntroduction to Particle Finder• Principle and requirementsPractical session• Demo with known sample• Customer samples: Bring your own samples!Practical session• Demo with known samplesCustomers samples: Bring your own samples! Presentation of the TERS technique• Principle and requirements • Application examplesDemo TERS• Presentation of the different tips and SPM modes • Laser alignment on the tip • T ERS spectra and TERS imaging on known samplesPractical session• Hands-on on demo samples (AFM mode)• Laser alignment on the tip • T ERS spectra and TERS imaging on known samplesRaman Options: DuoScan, Ultra Low Frequency, Particle Finder, TERS8Users of HORIBA Scientific Raman spectrometers who already understand the fundamentals of Raman spectroscopy and know how to use HORIBA Raman system and labSpec Software. It is adviced to participate in the basic Raman training first.Who should attendDates: February 14, 2019 June 27, 2019November 21, 2019Duration: 1 dayReference: RAM2From 9 am to 5:30 pm• Acquire theoretical and practical knowledge on SERS (Surface Enhanced Raman Spectroscopy)• Know how to select your substrate • Interpret resultsRaman SERSIntroduction to SERSPresentation of the SERS technique • Introduction: Why SERS?• What is SERS?• Surface Enhanced Raman basics • SERS substratesIntroduction to the SERS applications• Examples of SERS applications • Practical advice • SERS limitsDemo on known samplesCustomer samples: Bring your own samples!Raman Multivariate Analysis9Users of HORIBA Scientific Raman spectrometerswho already understand the fundamentals of Ramanspectroscopy and know how to use HORIBA Ramansystem and LabSpec Software. It is advised toparticipate in the basic Raman training first (RAM1).• Understand the Multivariate Analysis module• Learn how to use Multivariate Analysis for data treatment• Perform real case examples of data analysis on demo and customer dataIntroduction to Multivariate Analysis• Univariate vs. Multivariate analysis• Introduction to the main algorithms: decomposition (PCA and MCR), classification and quantification (PLS)Practical work on known datasets (mapping)• CLS, PCA, MCRIntroduction to classification• HCA, k-means• Demo with known datasetsIntroduction to Solo+MIA• Presentation of Solo+MIA Array• Demo with known datasetsData evaluation: cursors, CLS fitting, peak fitting• Fast mapping using SWIFT XSObjective: Being able to select the good parameters for Raman imaging and to perform data processScanning Probe Microscopy (SPM)• Instrumentation• T he different modes (AFM, STM, Tuning Fork) and signals (Topography, Phase, KPFM, C-AFM, MFM,PFM)Practical session• Tips and sample installation• Molecular resolution in AFM tapping mode• M easurements in AC mode, contact mode, I-top mode, KPFM• P resentation of the dedicated tips and additional equipment• O bjective: Being able to use the main AFM modes and optimize the parametersimaging)Practical session• Hands-on on demo samples (AFM mode)• Laser alignment on the tip• T ERS spectra and TERS imaging on known sample Day 3TERS Hands-on• T ERS measurements, from AFM-TERS tip installation to TERS mapping.• TERS measurements on end users samples.• Bring your own samples!28Practical informationCourses range from basic to advanced levels and are taught by application experts. The theoretical sessions aim to provide a thorough background in the basic principles and techniques. The practical sessions are directed at giving you hands-on experience and instructions concerning the use of your instrument, data analysis and software. We encourage users to raise any issues specific to their application. At the end of each course a certificate of participation is awarded.Standard, customized and on-site training courses are available in France, G ermany, USA and also at your location.Dates mentionned here are only available for HORIBA France training center.RegistrationFill in the form and:• Emailitto:***********************• Or Fax it to: +33 (0)1 69 09 07 21• More information: Tel: +33 (0)1 69 74 72 00General InformationThe invoice is sent at the end of the training.A certificate of participation is also given at the end of the training.We can help you book hotel accommodations. Following your registration, you will receive a package including training details and course venue map. We will help with invitation letters for visas, but HORIBA FRANCE is not responsible for any visa refusal. PricingRefreshments, lunches during training and handbook are included.Hotel transportation, accommodation and evening meals are not included.LocationDepending on the technique, there are three locations: Longjumeau (France, 20 km from Paris), Palaiseau (France, 26 km from Paris), Villeneuve d’Ascq (France 220 km from Paris) or at your facility for on-site training courses. Training courses can also take place in subsidiaries in Germany or in the USA.Access to HORIBA FRANCE, Longjumeau HORIBA FRANCE SAS16 - 18 rue du canal91165 Longjumeau - FRANCEDepending on your means of transport, some useful information:- if you are arriving by car, we are situated near the highways A6 and A10 and the main road N20- if you are arriving by plane or train, you can take the train RER B or RER C that will take you not far from our offices. (Around 15 €, 150 € by taxi from Charles de Gaulle airport, 50 € from Orly airport).We remain at your disposal for any information to access to your training place. You can also have a look at our web site at the following link:/scientific/contact-us/france/visi-tors-guide/Access to HORIBA FRANCE, Palaiseau HORIBA FRANCE SASPassage Jobin Yvon, Avenue de la Vauve,91120 Palaiseau - FRANCEFrom Roissy Charles de Gaulle Airport By Train • T ake the train called RER B (direction Saint RemyLes Chevreuse) and stop at Massy-Palaiseaustation• A t Massy-Palaiseau station, take the Bus 91-06C or 91-10 and stop at Fresnel• T he company is a 5 minute walk from the station,on your left, turn around the traffic circle and youwill see the HORIBA building29 Practical InformationAround 150 € by taxi from Charles de Gaulle airport. From Orly Airport By Train• A t Orly airport, take the ORLYVAL, which is ametro line that links the Orly airport to the AntonyRER station• A t Antony station, take the RER B (direction StRemy Les Chevreuse) and stops at Massy-Palai-seau station• A t Massy-Palaiseau station, take the Bus 91-06C, 91-06 B or 91-10 stop at Fresnel• T he company is 5 minutes walk from the station,on your left, turn around the traffic circle and youwill see the HORIBA building• O r at Orly take the Bus 91-10 stop at Fresnel.The company is 5 minutes walk from the station,on your left, turn around the traffic circle and youwill see the HORIBA building. We remain at yourdisposal for any information to access to your trainingplace. You can also have a look at our web site at thefollowing link:/scientific/contact-us/france/visi-tors-guide/Around 50 € by taxi from Orly airport.Access to HORIBA FRANCE, Villeneuve d’Ascq HORIBA Jobin Yvon SAS231 rue de Lille,59650 Villeneuve d’Ascq - FRANCEBy Road from ParisWhen entering Lille, after the exit «Aéroport de Lequin», take the direction «Bruxelles, Gand, Roubaix». Immmediatly take the direction «Gand / Roubaix» (N227) and No «Bruxelles» (A27) Nor «Valenciennes» (A23).You will then arrive on the ringroad around Villeneuve d’Ascq. Take the third exit «Pont de Bois».At the traffic light turn right and follow the road around, (the road will bend left then right). About 20m further on you will see the company on the right hand side where you can enter the car park.By Road from Belgium (GAND - GENT)Once in France, follow the motorway towards Lille. After «Tourcoing / Marcq-en-Baroeul», follow on the right hand side for Villeneuve d’Ascq. Take the exit «Flers Chateau» (This is marked exit 6 and later exit 5 - but it is the same exit). (You will now be following a road parallel to the mo-torway) Stay in the middle lane and go past two sets of traffic lights; at the third set of lighte, move into the left hand lane to turn under the motorway.At the traffic lights under the motorway go straight, (the road shall bend left then right). About 20 m further you shall see the company on the right hand side where you can enter the car park.AeroplaneFrom the airport Charles de Gaulle take the direction ‘Ter-minal 2’ which is also marked TGV (high speed train); where you can take the train to ‘Lille Europe’.Train - SNCFThere are two train stations in Lille - Lille Europe or Lille Flandres. Once you have arrived at the station in Lille you can take a taxi for HORIBA Jobin Yvon S.A.S., or you can take the underground. Please note both train stations have stations for the underground.Follow the signs:1. From the station «Lille Flandres», take line 1, direction «4 Cantons» and get off at the station «Pont de bois».2. From the station «Lille Europe», take line 2, direction «St Philibert» and get off at the following station «Gare Lille Flandres» then take line 1, direction «4 Cantons» and get off at the station «Pont de Bois».BusBus n°43, direction «Hôtel de Ville de Villeneuve d’Ascq», arrêt «Baudoin IX».InformationRegistration: Fill inthe form and send it back by FAX or Email four weeks before beginning of the training.Registration fees: the registration fees include the training courses and documentation. Hotel, transportation and living expenses are not included except lunches which are taken in the HORIBA Scientific Restaurant during the training.Your contact: HORIBA FRANCE SAS, 16-18 rue du Canal, 91165 Longjumeau, FRANCE Tel: + 33 1 64 74 72 00Fax: + 33 1 69 09 07 21E-Mail:***********************Siret Number: 837 150 366 00024Certified ISO 14001 in 2009, HORIBA Scientific is engaged in the monitoring of the environmental impact of its activitiesduring the development, manufacture, sales, installation and service of scientific instruments and optical components. Trainingcourses include safety and environmental precautions for the use of the instrumentsHORIBA Scientific continues contributing to the preservation of theglobal environment through analysis and measuring technologymentisnotcontractuallybindingunderanycircumstances-PrintedinFrance-©HORIBAJobinYvon1/219。

附录UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at theSurface and in theBulkJing Zhang, Meijun Li, Zhaochi Feng, Jun Chen, and Can Li*State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences,P. O. Box 110, Dalian 116023, ChinaRecei V ed: September 16, 2005; In Final Form: No V ember 4, 2005Phase transformation of TiO2 from anatase to rutile is studied by UV Raman spectroscopy excited by 325and 244 nm lasers, visible Raman spectroscopy excited by 532 nm laser, X-ray diffraction (XRD), andtransmission electron microscopy (TEM). UV Raman spectroscopy is found to be more sensitive to the surfaceregion of TiO2 than visible Raman spectroscopy and XRD because TiO2 strongly absorbs UV light. Theanatase phase is detected by UV Raman spectroscopy for the sample calcined at higher temperatures thanwhen it is detected by visible Raman spectroscopy and XRD. The inconsistency in the results from the abovethree techniques suggests that the anatase phase of TiO2 at the surface region can remain at relatively highercalcination temperatures than that in the bulk during the phase transformation. The TEM results show thatsmall particles agglomerate into big particles when the TiO2sample is calcined at elevated temperatures andthe agglomeration of the TiO2 particles is along with the phase transformation from anatase to rutile. It issuggested that the rutile phase starts to form at the interfaces between the anatase particles in the agglomeratedTiO2 particles; namely, the anatase phase in the inner region of the agglomerated TiO2 particles turns out tochange into the rutile phase more easily than that in the outer surface region of the agglomerated TiO2 particles.When the anatase particles of TiO2 are covered with highly dispersed La2O3, the phase transformation inboth the bulk and surface regions is significantly retarded, owing to avoiding direct contact of the anataseparticles and occupying the surface defect sites of the anatase particles by La2O3.1. IntroductionTitania (TiO2) has been widely studied because of its uniqueoptical and chemical properties in catalysis,[1]photocatalysis,[2]sensitivity to humidity and gas,[3,4] nonlinear optics,[5]photoluminescence,[6]and so on. The two main kinds of crystalline TiO2,anatase and rutile, exhibit different physical and chemicalproperties. It is well-known that the anatase phase is suitablefor catalysts and supports,[7]while the rutile phase is used foroptical and electronic purposes because of its high dielectricconstant and high refractive index.[8]It has been well demonstratedthat the crystalline phase of TiO2 plays a significant rolein catalytic reactions, especially photocatalysis.[9-11]Some studieshave claimed that the anatasephase was more active than therutile phase in photocatalysis.[9,10]Although at ambient pressure and temperature the rutile phaseis morethermodynamically stable than the anatase phase,[12]anatase is the common phase rather than rutile because anataseis kinetically stable in nanocrystalline TiO2at relatively lowtemperatures.[13] It is believed that the anatase phase transformsto the rutile phase over a wide range of temperatures.[14]Therefore, understanding and controlling of the crystalline phaseand the process of phase transformation of TiO2 are important,though they are difficult.Many studies[13-31]have been done to understand the processof the phase transformation of TiO2. Zhang et al.[15]proposedthat the mechanism of the anatase-rutile phase transformationwas temperature-dependent according to the kinetic data fromX-ray diffraction (XRD). On the basis of transmission andscanning electron microscopies, Gouma et al.[16] suggested thatrutile nuclei formed on the surface of coarser anatase particlesand the newly transformed rutile particles grew at the expenseof neighboring anatase particles. Penn et al.[17]suggested thatthe formation of rutile nuclei at twin interfaces of anataseparticles heated hydrothermally.Catalytic performance of TiO2 largely depends on the surfaceproperties, especially the surface phase, because catalyticreaction takes place on the surface. The surface phase of TiO2should be responsible for its photocatalytic activity because notonly the photoinduced reactions take place on the surface[32] butalso the photoexcited electrons and holes might migrate throughthe surface region. Therefore, the surface phase of TiO2, whichis exposed to the light source, should play a crucial role inphotocatalysis. However, the surface phase of TiO2, particularlyduring the phase transformation, has not been investigated. Thechallenging questions still remain: is the phase in the surfaceregion the same as that in the bulk region, or how does thephase in the surface region of TiO2 particle change during thephase transformation of its bulk? The difficulty in answeringthe above questions was mainly due to lacking suitabletechniques that can sensitively detect the surface phase of TiO2.UV Raman spectroscopy is found to be more sensitive tothe surface phase of a solid sample when the sample absorbsUV light.[33]We studied the phase transition of zirconia (ZrO2)from tetragonal phase to monoclinic phase by UV Ramanspectroscopy, visible Raman spectroscopy, and XRD.[33] Theseresults clearly indicated that the surface phase of ZrO2 is usuallydifferent from the bulk phase of ZrO2 and the phase transforma-tion of ZrO2 starts from its surface region and then graduallydevelops into its bulk when the ZrO2 with tetragonal phase iscalcined at elevated temperatures.These findings lead us to further investigate the phasetransformation in the surface region of TiO2 by UV Ramanspectroscopy as TiO2 also strongly absorbs UV light. In thisstudy, we compared the Raman spectra of TiO2 calcined atdifferent temperatures with excitation lines in the UV and visibleregions. XRD and transmission electron microscopy (TEM)were also recorded to understand the process of phase transformationof TiO2. It was found that the results of UV Ramanspectra are different from those of visible Raman spectra andXRD patterns. The anatase phase of TiO2 at the surface regioncan remain at relatively higher temperatures than that in thebulk at elevated calcination temperatures; namely, the anatasephase in the inner region of the agglomerated TiO2 particlesturns out to change into the rutile phase more easily than thatin the outer surface region of the agglomerated TiO2 particles.The literature[15,17,19] proposed the mechanism that phasetransformation of TiO2 might start at the interfaces of contactinganatase particles. If the anatase particles of TiO2 are separated,the phase transformation of TiO2 from anatase to rutile couldbe retarded or prohibited. Jing et al.[34]showed that La3+ did notenter the crystal lattices of TiO2 and was uniformly dispersedonto TiO2in the form of lanthana (La2O3) particles with smallsize. To verify the above assumption, this study also preparedthe anatase phase of TiO2 sample covered with La2O3 andcharacterized the above sample by visible Raman spectroscopyand UV Raman spectroscopy. The results of the two types ofRaman spectra are in agreement with each other and show thatthe TiO2 particle covered with La2O3 can retain its anatase phaseboth in the bulk and in the surface region even after calcinationat 900 °C.2. Experimental Section2.1. Catalyst Preparation.2.1.1. Preparation of TiO2. TiO2was prepared by precipitation method. To 100 mL of anhydrousethanol was added 20 mL of titanium(IV) n-butoxide(Ti(OBu)4). This solution was added to a mixture solution ofdeionized water and 100 mL of anhydrous ethanol. The molarratio of the water/Ti(OBu)4was 75. After the formed whiteprecipitate was stirred continuously for 24 h, it was filtered andwashed twice with deionized water and anhydrous ethanol.Finally, the sample was dried at 100 °C and calcined in air attemperatures from 200 to 800 °C for 4 h, and then cooled toroom temperature.2.1.2. Preparation of La2O3-Co V ered TiO2(La2O3/TiO2). Theabove TiO2powder calcined at 500 °C was used as a support.The critical La2O3 loading corresponding to monolayer coverageof La2O3 on the grain surface of TiO2 is 0.27 g/100 m2. [35,36] Onthe basis of the BET surface area of the TiO2 support (54.3m2/g), the monolayer dispersion capacity can also be expressedas 15 wt % La2O3 of the weight of TiO2. La2O3/TiO2 samples,containing different amounts of La2O3(0.5-6 wt %) wereprepared by a wet impregnation method. The support wasimpregnated with aqueous solution of various concentrationsof lanthanum nitrate (La(NO3)3·6H2O) andsubsequently stirredin a hot water bath until it was dried. After the sample waskept at 110 °C overnight, it was calcined at 900 °C in air for 4h. A TiO2 sample was prepared by calcining the TiO2supportat 900 °C for 4 h (denoted as TiO2-900) for comparison withthe La2O3/TiO2 sample. Pure La2O3was obtained by calciningLa(NO3)3·6H2O at 550 °C for 4 h.2.2. Characterization.2.2.1. UV Raman Spectroscopy. UVRaman spectra were measured at room temperature with a Jobin-Yvon T64000 triple-stage spectrograph with spectral resolutionof 2 cm-1. The laser line at 325 nm of a He-Cd laser wasused as an exciting source with an output of 25 mW. The powerof laser at the sample was about3.0 mW. The 244 nm linefrom a Coherent Innova 300 Fred laser was used as anotherexcitation source. The power of the 244 nm line at sample wasbelow 1.0 mW.2.2.2. Visible Raman Spectroscopy. Visible Raman spectrawere recorded at room temperature on a Jobin-Yvon U1000scanning double monochromator with the spectral resolutionof 4 cm-1. The line at 532 nm from a DPSS 532 Model 200532 nmsingle-frequency laser was used as the excitation source.2.2.3. X-ray Powder Diffraction (XRD), TEM, and Ultra V iolet-Visible Diffuse Reflectance Spectroscopy. XRD patterns wereobtained on a Rigaku MiniFlex diffractometer with a Cu KRradiation source. Diffraction patterns were collected from 20°to 80°at a speed of 5°/min. TEM was taken on a JEM-2011TEM for estimating particle size and morphology. UV-visdiffuse reflectance spectra were recorded on a JASCO V-550UV-vis spectrophotometer.2.2.4. Brunauer-Emmett-Teller (BET) Specific SurfaceArea. The BET surface area of the TiO2 support was measuredby nitrogen adsorption at 77 K using a Micromeritics ASAP2000 adsorption analyzer.3. Results3.1. Spectral Characteristics of Anatase and Rutile TiO2.The anatase and rutile phases of TiO2 can be sensitivelyidentified by Raman spectroscopy based on their Raman spectra.The anatase phase shows major Raman bands at 144, 197, 399,515, 519 (superimposed with the 515 cm-1 band), and 639cm-1.[37] These bands can be attributed to the six Raman-activemodes of anatase phase with the symmetries of Eg, Eg, B1g,A1g, B1g, and Eg, respectively.[37] The typical Raman bands dueto rutile phase appear at 143 (superimposed with the 144 cm-1band due to anatase phase), 235, 447, and 612 cm-1, whichcan be ascribed to the B1g, two-phonon scattering, Eg, and A1gmodes of rutile phase, respectively.38Additionally, the band at144 cm-1is the strongest one for the anatase phase and theband at 143 cm-1 is the weakest one for the rutile phase. PartsA and B, respectively, of Figure 1display the Raman spectra ofTiO2 calcined at 500 and 800 °C with excitation lines at 532,325, and 244 nm. Obviously, both visible Raman spectra andUV Raman spectra show that the TiO2 sample is in the anatasephase (Figure 1A) and rutile phase (Figure 1B).Figure 2 shows UV-vis diffuse reflectance spectra of theTiO2 sample calcined at 500 and 800 °C (the TiO2 sample is inthe anatase phase and rutile phase, respectively). For the anatasephase, the maximum absorption and the absorption band edgecan be estimated to be around 324 and 400 nm, respectively.The maximum absorption and the absorption band edge shiftto a little longer wavelength for the rutile phase.[39]By comparing the Raman spectra of the anatase (Figure 1A)or rutile phase (Figure 1B) excited by 532, 325, and 244 nmlines, it is found that the relative intensities of characteristicbands due to anatase or rutile phase in the high-frequency regionare different. For the anatase phase (Figure 1A), the band at638 cm-1 is the strongest one in the Raman spectrum with theexcitation line at 325 or 532 nm, while the band at 395 cm-1 isthe strongest one in the Raman spectrum with the excitationline at 244 nm.For the rutile phase (Figure 1B), the intensities of the bandsat 445 and 612 cm-1 are comparable in the visible Ramanspectrum. The intensity of the band at 612 cm-1 is strongerthan that of the band at 445 cm-1 in the Raman spectrum withthe excitation line at 325 nm, and the reverse is true for theRaman spectrum with the excitation line at 244 nm. In addition,for the rutile phase, a band at approximately 826 cm-1 appearsin the UV Raman spectra. Some investigations show that therutile phase of TiO2 exhibits a weak band at 826 cm-1 assignedto the B2g mode.[38,40]The fact that the relative intensities of the Raman bands ofanatase phase or rutile phase are different for UV Ramanspectroscopy and visible Raman spectroscopy are mainly dueto the UV resonance Raman effect because the laser lines at325 and 244 nm are in the electronic absorption region of TiO 2(Figure 2). There is no resonance Raman effect observed forthe TiO 2 sample excited by visible laser line, because the lineat 532 nm is outside the absorption region of TiO 2 (Figure 2).Therefore, for the anatase or rutile phase, the Raman spectroscopiccharacteristics in the visible Raman spectrum are differentfrom those in the UV Raman spectrum. When the UV laserline with different wavelengths is used as the excitation source,the resonance enhancement effect on the Raman bands ofanatase or rutile phase is different. For example, for the rutilephase (Figure 1B), the band at 612 cm -1 is easily resonanceenhanced when the excitation wavelength is 325 nm. Amongall the characteristic bands of the rutile phase, the extent ofresonance enhancement of 445 cm -1 is the strongest when the244 nm laser is used as the excitation source (Figure 1B).3.2. Semiquantitative Analysis of the Phase Compositionof TiO 2 by XRD and Raman Spectroscopy. The weightfraction of the rutile phase in the TiO 2 sample, W R, can beestimated from the XRD peak intensities using the followingformula:[41]]84.801[1)(rut ana A A W R +=where A ana and A rut represent the X-ray integrated intensities ofanatase (101) and rutile (110) diffraction peaks, respectively.To estimate the weight fraction of the rutile phase in the TiO 2sample by Raman spectroscopy, pure anatase phase and purerutile phase of the TiO 2 sample, which have been prepared bycalcination of TiO 2 powder at 500 and 800 °C for 4 h, weremechanically mixed at given weight ratio and ground carefullyto mix sufficiently. Figure 3A displays the visible Raman spectra of the mechanicalmixture with 1:1, 1:5, 1:10, 1:15, 5:1, and 10:1 ratios ofanatase phase to rutile phase. The relationship between the arearatios of the visible Raman band at 395 cm-1 for anatase phaseto the band at 445 cm -1 for rutile phase (A 395 cm -1/A 445 cm -1) andthe weight ratios of anatase phase to rutile phase (W A /W R ) isplotted in Figure 3B. It can be seen that a linear relationshipbetween the band area ratios and the weight ratios of anatasephase to rutile phase in the mixture is obtained. The rutilecontent in the Degussa P25, which usually consists of roughlyabout 80% anatase and 20% rutile phase,42 was estimated bythis plot. Our Raman result indicates that the rutile content inthe Degussa P25 is about 18.7%, which is close to the knownresult. Thus, the above linear relationship based on visibleRaman spectroscopy can be used to estimate the rutile contentin TiO 2.Figure 4A presents the UV Raman spectra of the mechanicalmixture with 1:1, 1:2, 1:4, 1:6, 1:10, and 1:15 ratios of anatasephase to rutile phase with the excitation line at 325 nm. Figure4B shows the plot of the area ratios of the UV Raman band at612 cm-1 for rutile phase to the band at 638 cm-1 for anatasephase (A 612 cm -1/A 638 cm -1) versus the weight ratios of rutile phaseto anatase phase (W R/W A). There is also a linear relationshipbetween the band area ratios and the weight ratios of rutile phaseto anatase phase.3.3. Phase Transformation of TiO 2 at Elevated CalcinationTemperatures.3.3.1. XRD Patterns and Visible Raman Spectraof TiO2Calcined at Different Temperatures. Figure 5 showsthe XRD patterns of TiO2calcined at different temperatures.The “A” and “R” in the figure denote the anatase and rutilephases, respectively. For the sample before calcination, diffractionpeaks due to the crystalline phase are not observed,suggesting that the sample is still in the amorphous phase. Whenthe sample was calcined at 200 °C, weak and broad peaks at2θ=25.5°, 37.9°, 48.2°, 53.8°, and 55.0° were observed. Thesepeaks represent the indices of (101), (004), (200), (105), and(211) planes of anatase phase, respectively.[43] These results suggest that some portions of the amorphous phase transforminto the anatase phase. The diffraction peaks due to anatasephase develop with increasing the temperature of calcination.When the calcination temperature was increased to 500 °C, thediffraction peaks due to anatase phase became narrow andintense in intensity. This indicates that the crystallinity of theanatase phase is further improved.[44]When the sample was calcined at 550 °C, weak peaks wereobserved at 2θ=27.6°, 36.1°, 41.2°, and 54.3°, whichcorrespond to the indices of (110), (101), (111), and (211) planesof rutile phase. [43]This indicates that the anatase phase starts totransform into the rutile phase at 550 °C. The diffraction peaksof anatase phase gradually diminish in intensity and thediffraction patterns of rutile phase become predominant withthe calcination temperatures from 580 to 700 °C. These resultsclearly show that the phase transformation from anatase to rutileprogressively proceeds at the elevated temperatures. The diffractionpeaks assigned to anatase phase disappear at 750 °C,indicating that the anatase phase completely changes into therutile phase. The diffraction peaks of rutile phase became quitestrong and sharp after the sample was calcined at 800 °C, owingto the high crystallinity of the rutile phase.Figure 6 displays the visible Raman spectra of TiO2 calcinedat different temperatures. For the sample before calcination, twobroad bands at about 430 and 605 cm-1are observed, indicatingthat the sample is in the amorphous phase.[19]For the samplecalcined at 200 °C, a Raman band at 143 cm-1 is observed andthe high-frequency region shows interference from the fluorescencebackground, which might come from organic species.After calcination at 300 °C, other characteristic bands of anatasephase appear at 195, 395, 515, and 638 cm-1, but some portionsof the sample may exist in the amorphous phase because thereis still a broad background in Figure 6.It was found that, when the sample was calcined at 400 °C,the fluorescence disappeared, possibly because the organicresidues were removed by the oxidation. The bands of anatasephase increased in intensity and decreased in line width whenthe sample was calcined at 500 °C. This result suggests thatthe crystallinity of the anatase phase is greatly improved,[18] whichis confirmed by XRD (Figure 5). The enlarged section of Figure6 shows the Raman spectrum of the high-frequency region ofthe sample calcined at 500 °C. Besides the bands at 395, 515,and 638 cm-1, two very weak bands at 320 and 796 cm-1areobserved. These two bands can be assigned to a two-phononscattering band and a first overtone of B1g at 396 cm-1,respectively. [37]It is noteworthy that a very weak band appearsat 445 cm-1 due to rutile phase for the sample calcined at 550°C. This indicates that the anatase phase starts to change intothe rutile phase at 550 °C. This result is in good agreementwith that of XRD patterns (Figure 5). The weightpercentageof the rutile phase in the samples calcined at different temperatureswas estimated by visible Raman spectroscopy and XRD(shown in Figure 7). As seen from Figure 7, the rutile contentestimated from visible Raman spectrum and XRD pattern ofthe sample calcined at 550 °C is 4.2% and 5.7%, respectively.It can be seen that the rutile contents estimated by visible Ramanspectroscopy and XRD are also in accordance with each other.When the sample was calcined at 580 °C, other twocharacteristic bands were observed at 235 and 612 cm-1 due toRaman-active modes of rutile phase. Figure 7 shows the rutilecontent is 13.6% and 10.9% based on the visible Ramanspectrum and XRD pattern of the sample calcined at 580 °C.The intensities of the bands of rutile phase (235, 445, and 612cm-1) increased steadily while those of the bands of anatasephase (195, 395, 515, and 638 cm-1) decreased when thecalcination temperatures were elevated from 600 to 680 °C(Figure 6). These results suggest that the TiO2 sample undergoesthe phase transformation from anatase to rutile gradually. Therutile content was estimated for the samples calcined from 600to 680 °C based on the visible Raman spectra. The results showthat the content of the rutile phase is increased from 33.1% to91.2% respectively for the samples calcined at 600 and 680 °C(Figure 7). The XRD results corresponding to the above twosamples indicate that the rutile content is changed from 32.9%to 90.7% (Figure 7). These results clearly show that the rutilecontent in the sample estimated by visible Raman spectroscopyis agreement with that estimated by XRD.The Raman spectrum of the sample calcined at 700 °C showsmainly the characteristic bands of rutile phase, but the very weakbands of anatase phase are still observed (Figure 6). When thesample was calcined at 750 °C, the bands of anatase phasedisappeared and only the bands due to rutile phase (143, 235,445, and 612 cm-1) were observed. These results indicate thatthe anatase phase completely transforms into the rutile phaseand are consistent with the results from XRD (Figure 5). Whenthe temperature was increased to 800 °C, the characteristic bandsdue to rutile phase increased in intensity further.Both the results of XRD and visible Raman spectra (Figures5 and 6) show that the anatase phase appears at around 200 °Cand perfect anatase phase is formed after calcination at temperaturesof 400-500 °C. The rutile phase starts to form at 550°C, and the anatase phase completely transforms into the rutilephase at 750 °C. The signals of visible Raman spectra comemainly from the bulk region of TiO2because the TiO2 sampleis transparent in the visible region (Figure 2).[33]XRD is knownas a bulk-sensitive method. Therefore, it is essentially inagreement between the results of visible Raman spectra andXRD patterns.3.3.2. UV Raman Spectra of TiO2 Calcined at DifferentTemperatures. UV-vis diffuse reflectance spectra (Figure 2)clearly show that TiO2 has strong electronic absorption in theUV region. Thus, the UV Raman spectra excited by a UV laserline contain more signal from the surface skin region than thebulk of the TiO2 sample because the signal from the bulk isattenuated sharply due to the strong absorption.[33] Therefore, ifa UV laser line in the absorption region of TiO2is used as theexcitation source of Raman spectroscopy, the information fromUV Raman spectra is often different fromthat of visible Ramanspectra.The laser line at 325 nm was selected as the excitation sourceof the UV Raman spectra. The UV Raman spectra and thecontent of the rutile phase of the TiO2 sample calcined atdifferent temperatures are shown in parts A and B, respectively,of Figure 8. When the sample was calcined at 200 or 300 °C,the Raman band at 143 cm-1 with a shoulder band at 195 cm-1and three broad bands at 395, 515, and 638 cm-1 were observed,indicating that the anatase phase is formed in the sample.However, the low intensity and the broad band indicate thatthe amorphous phase still remains in the sample. It can be seenthat the fluorescence in the high-frequency region can be avoidedwhen the UV laser line is used as the excitation line. However,the corresponding visible Raman spectra (Figure 6) showinterference from the fluorescence.All bands assigned to anatase phase become sharp and strongafter calcination at 500 °C (Figure 8A). These results are inagreement with those of XRD and visible Raman spectra(Figures 5 and 6). The UV Raman spectra of the sample withthe calcination temperatures from 550 to 680 °C are essentiallythe same as those of the sample calcined at 500 °C (Figure 8A).However, according to the XRD patterns and visible Ramanspectra (Figures 5 and 6), the anatase phase starts to transforminto the rutile phase at only 550 °C and the anatase phasegradually changes into the rutile phase in the temperature rangeof 550-680 °C.After calcination at 700 °C, a new band at 612 cm-1 andtwo weak bands at 235 and 445 cm-1 due to rutile phase appearwhile the intensities of the bands of anatase phase begin todecrease (Figure 8A). On the basis of the UV Raman spectrumand XRD pattern of the sample calcined at 700 °C, the rutilecontent in the sample is 56.1% and 97.0%, respectively (Figure8B). It is found that the rutile content estimated by UV Ramanspectroscopy is far less than that estimated by XRD.When the sample was calcined at 750 °C, the intensities ofthe bands due to rutile phase increased, but the intensities ofthe bands due to anatase phase were still strong in the UVRaman spectra (Figure 8A). The UV Raman spectrum of thesample calcined at 750 °C indicates that the rutile content is84.3% (Figure 8B). However, the results of XRD and visibleRaman spectrum (Figures 5 and 6) suggest that the anatase phasetotally transformed into the rutile phase after the sample wascalcined at 750 °C. The characteristic bands due to anatase phasedisappear and the sample is in the rutile phase after calcinationat 800 °C (Figure 8A). Obviously, there are distinct differences between the results from the UV Raman spectra, visible Ramanspectra, and XRD patterns. It seems that the anatase phaseremains at relatively higher temperatures when detected by UVRaman spectroscopy than by XRD and visible Raman spectroscopy. Another UV laser line at 244 nm was also selected as theexcitation source of UV Raman spectroscopy in order to getfurther insights into the phase transformation of TiO2. Theresults of the UV Raman spectra of TiO2 calcined at differenttemperatures with the excitation line at 244 nm are presentedin Figure 9. When the sample was calcined at 200 °C, four broadbands were observed at 143, 395, 515, and 638 cm-1, whichclearly indicate that the anatase phase exists in the sample. Theintensities of the Raman bands due to anatase phase (143, 395,515, and 638 cm-1) become strong after calcination at 500 °C.The UV Raman spectra hardly change for the sample calcinedatdifferent temperatures even up to 680 °C. The characteristicbands (445 and 612 cm-1) of rutile phase appear only whenthe calcination temperature exceeds 700 °C. This result is ingood agreement with that from the UV Raman spectrum of thesample calcined at 700 °C with 325 nm excitation (Figure 8A).The intensities of the bands due to anatase phase (395, 515,and 638 cm-1) decrease while those of bands assigned to rutilephase (445 and 612 cm-1) increase after calcination at 750 °C(Figure 9). When the sample was calcined at 800 °C, the Ramanbands due to anatase phase disappeared, while the bands of rutilephase developed. This result indicates that the sample calcinedat 800 °C is in the rutile phase. It is interesting to note that theresults of the UV Raman spectra with the excitation lines at325 and 244 nm are in agreement with each other but aredifferent from those of XRD patterns and visible Raman spectra.3.3.3. TEM of the TiO2 Sample Calcined at DifferentTemperatures. TEM was used to characterize the microstructureof the TiO2 sample calcined at 500, 600, and 800 °C (shown inFigure 10). Most particles in the sample calcined at 500 °Cexhibit diameters in a range between 10 and 30 nm (Figure10a). On the other hand, remarkable agglomeration is observedfor the TiO2 sample calcined at 500 °C. The particle sizeincreases after calcination at 600 °C (Figure 10b). Accordingto the results of XRD and visible Raman spectra (Figures 5and 6), the sample undergoes the phase transformation fromanatase to rutile gradually in the temperature range of 550-680 °C. These results imply that the phase transformation andgrowth of the particle size are interrelated. Many researchers[45-49]reported similar phenomena. Kumar et al.45 attributed thisparticle size growth to the higher atomic mobility because ofbond breakage during the phase transformation. When thecalcination temperature was increased to 800 °C, TiO2 particlesfurther grew and the particle size could be as large as about200 nm (Figure 10c).3.4. Visible Raman Spectra and UV Raman Spectra ofLa2O3/TiO2 with Increasing La2O3 Loading. The literature[15,17,29]proposed the mechanism that the phase transformationof TiO2might start at the interfaces of contacting anataseparticles. If direct contact between anatase particles of TiO2 isavoided, the phase transformation of TiO2 from anatase to rutilecould be retarded or prohibited. This assumption may be verifiedby covering the surface of anatase TiO2 with an additive. Inthis work, we used La2O3dispersed on anatase TiO2to preventthe anatase particles from contacting directly because it wasreported that La2O3could be highly dispersed on anataseTiO2.[34-36]Figure 11A displays the visible Raman spectra of La2O3/TiO2with increasing La2O3 loading. TiO2 support is in the anatasephase because only characteristic bands (143, 195, 395, 515,and 638 cm-1) due to anatase phase are observed. When theTiO2 support was calcined at 900 °C (TiO2-900), the Ramanspectrum gave the characteristic bands of rutile phase, indicatingthat the TiO2-900 sample was in the rutile phase. When thesample with 0.5 wt % La2O3 loading was calcined at 900 °C,the Raman spectrum drastically changed, as compared to theTiO2-900 sample. Only characteristic bands of anatase phasewere observed, suggesting that the TiO2 sample retains itsanatase phase when La2O3 loading is 0.5 wt % while the TiO2-900 sample is in the rutile phase. The。

硝酸钠分析报告1. 简介硝酸钠(NaNO3)是一种常见的无机化合物,具有广泛的应用领域。
本报告旨在通过对硝酸钠样品的分析,了解其化学性质、物理性质以及分析方法。
2. 化学性质硝酸钠是无色结晶固体,易溶于水。
在水溶液中,硝酸钠完全解离为钠离子(Na+)和亚硝酸根离子(NO3-)。
硝酸钠具有氧化性和还原性,在一些化学反应中起到很重要的作用。
3. 物理性质硝酸钠的相对分子质量为85.0 g/mol,密度为2.26 g/cm3。
它在室温下为无色结晶固体,熔点为308℃,沸点为380℃。
硝酸钠的溶解度与温度密切相关,随着温度的升高,溶解度也增加。
4. 分析方法4.1 硝酸钠含量测定硝酸钠的含量可以通过酸碱滴定法进行测定。
具体步骤如下:1.取一定量的硝酸钠样品,并加入适量的酸性溶液进行酸化处理。
2.将酸化后的样品用标准酸溶液进行滴定,直到溶液由红变黄为止。
3.记录所耗酸溶液的体积,并根据滴定反应的化学计量关系计算硝酸钠的含量。
4.2 硝酸钠纯度测定硝酸钠样品的纯度可以通过热重分析进行测定。
具体步骤如下:1.取一定量的硝酸钠样品,并将其放入热重仪中进行加热。
2.在一定的温度范围内,观察样品的质量变化情况。
3.根据样品的质量损失情况,计算硝酸钠样品的纯度。
5. 结论通过以上的分析方法,我们可以得出以下结论:1.硝酸钠是一种常见的无机化合物,具有氧化性和还原性。
2.硝酸钠的物理性质包括密度、熔点和沸点等。
3.硝酸钠的含量可以通过酸碱滴定法进行测定。
4.硝酸钠的纯度可以通过热重分析进行测定。
在实际应用中,对硝酸钠样品进行分析可以确保其质量和纯度,从而满足不同领域的需求。
6. 参考文献1.Zhang, Y., Liu, D., Yu, F., & Li, H. (2018). Spectroscopic investigations ofthe interactions between bovine serum albumin and sodium nitrate.Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 188,433-440.2.Zhang, Y., Li, H., Xu, Y., & Yu, F. (2019). Raman spectroscopicinvestigation of the interactions between sodium nitrate and bovine serumalbumin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 207, 48-54.。
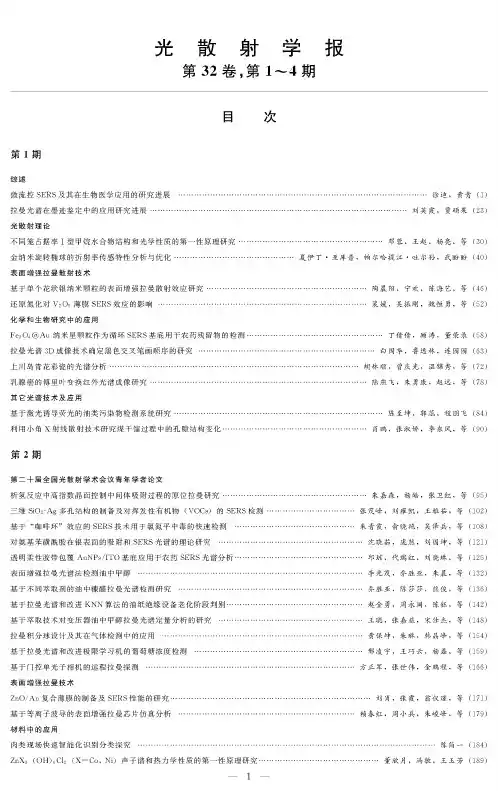
光散射学报第32卷,第#〜4期目次第1期综述微流控SERS及其在生物医学应用的研究进展..................拉曼光谱在墨迹鉴定中的应用研究进展..........................光散射理论不同笼占据率I型甲烷水合物结构和光学性质的第一性原理研究…金纳米旋转椭球的折射率传感特性分析与优化...................表面增强拉曼散射技术基于单个花状银纳米颗粒的表面增强拉曼散射效应研究..........还原氮化对V2O5薄膜SERS效应的影响........................化学和生物研究中的应用Fe3O4@Au纳米星颗粒作为循环SERS基底用于农药残留物的检测拉曼光谱&D成像技术确定黑色交叉笔画顺序的研究............上川岛青花彩瓷的光谱分析.....................................乳腺癌的傅里叶变换红外光谱成像研究..........................其它光谱技术及应用基于激光诱导荧光的油类污染物检测系统研究...................利用小角X射线散射技术研究煤干幅过程中的孔隙结构变化.........................................徐迪,黄青⑴..............................刘英霞!贾硕果(23).......................邓蓉!王赵!杨亮!等(30)夏伊丁-亚库普,帕尔哈提江-吐尔孙,武盼盼(40)...................陶晨阳,宁欢,陈海艺,等(46)...................裴媛!吴振刚,魏恒勇!等(52).......................丁倩倩!顾涛!董荣录(51).....................白国华,鲁逸林,连园园(63)................胡林顺,曾庆光,温锦秀,等(72)...................陆燕飞!朱勇康!赵远!等(71).......................陈至坤,郭蕊,程朋飞(14)...................肖鹏!张淑娇,李东风!等(50)第2期第二十届全国光散射学术会议青年学者论文析应中面控中的原拉曼研究……………………………………………三维SiO2-Ag多孔结构的制备及对挥发性有机物(VOC:)的SERS检测........................基于“咖啡环$效应的SERS技术用于氯氮平中毒的快速检测.................................对氨基苯磺酰胺在银表面的吸附和SERS光谱的理论研究...................................... 透明柔性胶带包覆AuNPs/ITO基底应用于农药SERS光谱分析.................................表面增强拉曼光谱法检测油中甲醇............................................................基于不同萃取剂的油中糠醛拉曼光谱检测研究.................................................基于拉曼光谱和改进KNN算法的油纸绝缘设备老化阶段判别...................................基于萃取技术对变压器油中甲醇拉曼光谱定量分析的研究...................................... 拉曼分球及其在中的应用………………………………………………………………基于拉曼光谱和改进极限学习机的葡萄糖浓度检测............................................基于门控单光子相机的远程拉曼探测..........................................................表面增强拉曼技术ZnO/Au复合薄膜的制备及SERS性能的研究...................................................基于等离子波导的表面增强拉曼芯片仿真分析.................................................…朱嘉森,杨皓,张卫红,等(95)张茂峰,刘雍凯,王雅茹,等(102)朱青霞,,吴,等101,,刘国坤,等121 -■邱琼,代瑞红,刘晓琳,等125 -■李光茂,乔胜亚,朱晨,等132 -■乔胜亚,陈莎莎,熊俊,等136 -■赵金勇,周永阔,陈,等142王,张嘉,,等141 -■黄保坤,朱琳,,等154,王,杨,等159,张,金鹏程,等166••…刘肖,张霞,,等171,,朱,等179材料中的应用肉类现场快速智能化识别分类探究陈简一(114)ZnX&(OB))C12(X=Co,Ni)声子谱和热力学性质的第一性原理研究.................................董欣月,冯敏,王玉芳(119)第3期综述光声成像技术表面增强拉曼散射技术基于拉曼光谱相似度比对的油纸绝缘老化阶段诊断花粉的表面增强拉曼光谱鉴别研究Au@PS阵列SERS基底的特性研究表面增强拉曼光谱对抗肿瘤药物5-氟尿的检测研究蒋文萍,吴其鑫,,等(195%张腾翼,古亮,陈等(202…符致秋,刘刚,,等(210裴君妍,徐宗伟,王,等(217周国良,黄光耀,李盼,等(224化学和生物研究中的应用取代基对观es o-四苯基CD几何结构,红外拉曼振动频率及电子光谱的影响的理论研究张坚,李秀(230) PX装置吸附塔进料非芳含量的拉曼定量分析蒋飘逸,戴连奎(237)光散射理论基于光散射理论的玻璃晶圆表面缺陷检测方法研究涂政乾,董立超,赵东峰,等(245)对称性破缺纳米核壳二聚体的近场特性研究姜继玉,姜莎莎,吕靖薇,等(251)氮化_低温高压光谱研究范春梅,刘静仪,刘珊,等(259)材料中的应用嵌入金属纳米颗粒提高晶硅薄膜太阳能电池吸收率肖亮,朱群志(266)其它光谱技术及应用一种新的布里渊光时域定位技术蒋超(274)光谱法在唐卡蓝、绿色颜料分析中的应用张蕊,方小济,巨建伟(280)基于激光拉曼光谱快速无损检测牛油果油的研究张凤娟,黄敏,刘振方(288)第4期综述气溶胶单颗粒的拉曼测量方法,张(295)表面增强拉曼散射技术基于等离子波导的表面增强拉曼芯片仿真分析赖春红,周小兵,朱峻峰,等(301)单链DNA表面增强拉曼散射检测条件的优化蒋承顺,李旺,柳艳,等(306)基于SERS阵列的铜绿假单胞菌代谢产物铜绿菌素即时检测陈燕,陈欢,陈周恬,等(312)三氧化鸭超薄纳米片SERS活性基底的制备与性能研究孙宗杰,林东岳,何遥,等(320)材料中的应用小角X射线散射法研究枣树的微孔结构武海娟,翟红生,杨春明,等(328)化学和生物研究中的应用基于等离子体四极共振的金纳米长方体颗粒的折射率传感性能研究刘静雅,张现周(335)胆酸钠自组装行为的小角X射线散射研究李博楠,李天富,刘荣灯,等(343)金纳米颗粒在全血环境中近场增强特性李俊平,陈娜,刘书朋,等(348)仪器和方法学消光光谱颗粒粒径测量方法影响因素实验研究杨斌,赵蓉,王继,等(355)自组装纳米球热压印制备金属纳米盘阵列的研究曹燕,陈溢杭(361)其它光谱技术及应用江苏仪征刘集联营西汉墓岀土彩绘陶俑颜料分析范陶峰(369)陕西绥德县博物馆馆藏青铜甑锈蚀物的拉曼光谱分析付倩丽,康卫东,张尚欣,等(375)原位ATR-IR光谱研究钳酸钠光催化全分解水机理丁倩,陈涛,冯兆池,等(381) L-半胱氨酸功能化的碳量子点为探针快速检测花旗松素程家维,张宇辉,杨季冬(386)THE JOURNAL OF LIGHT SCATTERINGVol.32No.#〜4CONTENTSNo.1OverviewMicrofluidic SERS form and its Biomedical Applications..................................................................................................XU Di&HUANG Qing(1) Research Progress and Application of Raman Spectroscopy on the Identification of Ink Marks................LIU Yingxia,51A Shuoguo(23) Theories of Light ScatteringStudies on the Structure and Optical Properties of Different Cage Occupancy on si Methane Hydrate by First-principle ............................................................................................................................................DENGRong,WANG Zhao&YANG Liang&etal(30) Analysis and Optimization of Refractive Index Sensing of Gold Nanospheroid.YAKUPU Xiayiding,TUERSUN Paerhatijiang,WU Panpan(40)Surface-Enhanced Raman Scattering(SERS)Surface-enhanced Raman Scattering Study of Individual Flower-like Silver Nanoparticles....................................................................................................................................TAO Chenyang,NING Huan,CHEN Haiyi,et al(46) The Impact of Reduction Ntridation on SERS Effect of V2O5Film....................PEI Yuan,WU Zhengang,WEI Hengyong,et al(52) Application in Chemistry and Biology ResearchesFe3O4@Au nanostar as reproducible SERS substrates for the detection of pesticide residues.....................................................................................................................................................DING Qianqian&GU Tao&DONG Ronglu(58) Study of Raman Spectroscopy3D Profilometry to Determine the Sequence of Black Pen Ink Crossings.....................................................................................................................................................BAI Guohua,LU Yilin,LIAN Yuanyuan(63) Spectral Analysis of Blue-and-Whte porcelain on Shangchuan Island............HU Linshun&ZENG Qingguang&WEN Jiniu&et al(72) Study on Breast Cancer by Fourier Transform Infrared Spectroscopy Imaging••-LU Yanfei,ZHU Yongkang,ZHAO Yuan,et al(78) Other Optical Spectroscopic Techniques and ApplicationsResearch on Oil Contaminant Detection System Based on Laser Induced Fluorescence.................................................................................................................................................CHEN Zhikun&GUO Rui&CHENG Pengfei(84) Study on Pore Structural Changes of Coal Carbonization by Small Angle X-ray Scattering Technology...................................XIAO Peng,ZHANG Shujiao&LI Dongfeng,et al(90)No.2Papers by young scholars at the20th National Conference on light scattering=nsituRamanEvidenceof=ntermediatesAdsorptionEngineeringby High-=ndexFacetsControlduring HydrogenEvolutionReaction ....................................................................................................................................ZHU Jia s en,YANG Hao,ZHANG Weihong,et al(95) PreparationofThree-Dimensional(3D)SiO2-AgPorousStructureandSERSDetectionofVolatileOrganicCompounds(VOCs) ...............................................................................................................................ZHANG Mao f e ng&LIU Yongkai&WANG Yaru&et al(102) Antipsychoticdrugpoisoning monitoringofclozapineinurinebyusingco f eeringe f ectbasedsurface-enhancedRamanspectroscopy ............................................................................................................................................ZHU Qingxia&YU Xiaoyan&WU Ze'bing&et al(108) TheoreticalStudyofAdsorptionandSERSSpectraofSulfanilamideonSilverSurfaces........................................................................................................................................SHEN Xiaoru&PANG Ran&LIU Guokun&et al(121) Transparent flexible tape coated AuNPs/ITO substrate for pesticide SERS spectral analysisQIUQiong,DAIRuihong,LIU Xiaolin,tal(125) Detection of Methanol in Oil by Surface-enhanced Raman Spectroscopy...........LI Guangmao,QIAO Shengya,ZHU Chen,et al(132) RamanSpectroscopicDetectionofFurfuralinOilBasedonDi f erentExtractants....................................................................................................................................QIAO Shengya,CHEN Shasha,XIONG Jun,et al(136) Aging Stage Discrimination of Oil-Paper Insulation Equipment Based on Raman Spectrum and Improved KNN AlgorthmsZHAO Jinyong&ZHOU Yongkuo&CHEN Yu&tal(142) Study on Quanttative Analysis Method of Methanol Raman Spectra in OilBy Extraction TechnologyWANG Cong&ZHANGJiayi&SONG Shiji&tal(148)TheDesignofRamanIntegratingSphereandtheApplicationofDetectingGasesHUANGBaokun&ZHULin&HANJingf ng&tal(154)GlucoseconcentrationdetectionbasedonRamanspectroscopyandimprovedExtremeLearning MachineXING Lingyu&WANG Qiaoyun&YANG L i&tal(159) Remote Raman Detection Based on Gated-Single-Photon Camera......FANG Zhengjun&ZHANG ShPei&Jin Pengcheng&et al(166) Surface-EnhancedRamanSca t ering(SERS)Fabricationand SERS Properties Studies of ZnO/Au Composite film..........................LIU Xiao&ZHANG Xia&WENG Yijin&et al(171) Simulation AnalysisoJSurJace-enhancedRamanSca t eringChipBasedonthePlasma WaveguideI Chunhong,ZHOU Xiaobin,ZHU Jun f e ng,et al(179)FastInte l igentIdentiicationandExplorationoJMeatOnSite..........................................................................................................CHENJianyi(184)OtherOpticalSpectroscopicTechniquesandApplicationsFirst-principles Study of Phonon Spectra and Thermodynamic Properties of Z11X3(OB))C12(X=Co,Ni).................................................................................................................................................DONG Xinyue,FENG Min&WANG Yufang(119) No.3OverviewPhotoacoustic imaging................................................................................................................J1ANG Wenping&WU Qixin&MIN Jun&e=al(195) Surface-Enhanced Raman Scattering(SERS)Aging Phase Diagnosis of Oil Paper Insulation Based on Raman Spectral Similarty Ratio...............................................................................................................................ZHANG Tengyi,GU Liang,CHEN Xingang,et al(202) Discrimination of Pollen by Surface Enhanced Raman Spectroscopy......................................FUZhiqiu&LIU Gang&AN Ran&et al(210) Study on SERS substrate properties ofAu@PS arrays................................................................................PEI Junyan&XUZongwei,et al(217) Detection of5-Fluorouracil by Surface Enhanced Raman Spectroscopy ZHOU Guoliang,HUANG Guangyao&LI Pan,et al(224) Application in Chemistry and Biology ResearchesInfluences by substtuents on the geometrical structure&infrared and raman vibration frequency and electronic spectrum forme s o-tetraphenylporphyrin:A theoretical study................................................................................................................ZHANG Jian,Li Xiu(230) Quantitative Analysis for Non-aromatic Hydrocarbon in the Feedstock of an Adsorption Tower in a P-Xylene Unit Based on Raman Spectroscopy....................................................................................................................................................................................JIANG Piaoyi,DAI Liankui(237) Theories of Light ScatteringResearch on Surface Defect Detection Methodof Glass Wafer Based on Light Scattering Theory...............................................................................................................................Tu Zhengqian&Dong Lichao,Zhao Dongfeng&et al(245) ResearchonNearFieldCharacteristicsofSymmetryBreaking multilayerednanoshe l sdimer....................................................................................................................................JIANG Jiyu,JIANG Shasha,LV Jingwei,et al(251) Low-temperature and high-pressure Spectroscopy study of Gallium N t ride............FAN Chunme i,LIU Jingyi,LIU Shan,e t al(259) Other Optical Spectroscopic Techniques and ApplicationsEmbedding Metal NanEparticles tEIncrease the AbsErptiEn RateEf Crysta l ine SilicEn Thin Film SElar Ce l s...............................................................................................................................................................................XIAO Liang,ZHU Qunzhi(266) OtherOpticalSpectroscopicTechniquesandApplicationsA new Brillouin optical time domain localization technique................................................................................................................JIANG Chao(274) ApplicaNionofSpecNromeNryon AnalysisofBlueandGreenPigmenNsinNheThangka.....................................................................................................................................................ZHANG Rui,FANG Xiaoji,JU Jianwei(210) SNudyonRapid Non-desNrucNiveTesNingofAvocadoOilBasedonLaserRamanSpecNroscopy....................................................................................................................................ZHANG Fengjuan&HUANG Min&LIUZhenfang(211) No.4OverviewRaman Spectroscopy Measurement for Aerosol Single Particle.....................................................CHANG Pianpian&ZHANG Yunhong(295) Surface-EnhancedRamanSca t ering(SERS"Simulation AnalysisoJSurJace-enhancedRamanSca t eringChipBasedonthePlasma WaveguideI Chunhong&ZHOU Xiaobin&ZHU Jun f e ng&et al(301) OptimizationoJsurJaceenhancedRamansca t eringdetectionconditionsJorsingle-strandedDNAJIANGCh ngshun,LIWang,LIUYan,tal(306" SERS array toward point-of-care detection of Pseudomonas aeruginosa metabolteCHEN Yan,CHEN Huan,CHEN Zhoutian,tal(312" Preparation of Tungsten Trioxide Ultrathin Nanosheets for SERS Active Substrate and Its Performance............................................................................................................................................SUN Zongjie,LIN Dongyue,HE Yao,et al(320) OtherOpticalSpectroscopicTechniquesandApplicationsSAXS Study on the Micropore Structure of Jujube...................................WU Haijuan,ZHAI Hongsheng,YANG Chunming,et al(321) ApplicationinChemistryandBiologyResearchesStudyEftheRefractiveIndexSensingPerfErmancesEfAu NanEcubEidParticlesBasedEnthePlasmEnicQuadrupEleResEnance ......................................................................................................................................................................LIU Jingya&ZHANG Xianzhou(335) StudyEnself-assemblybehaviErEfsEdiu34)Near-fieldEnhancementCharacteristicsEfGEld NanEparticlesin WhEleBlEEdEnvirEnmentLI Junping&CHEN Na&LIUShup ng&tal(341) Instruments and MethodsExperimentalResearchEnInfluenceFactErsEfParticleSize MeasurementMethEdbasedEnExtinctiEnSpectrEscEpy .................................................................................................................................................YANG Bin&ZHAORong,WANG Ji,e t al(355) StudyEnfabricatiEnEfMeta l icNanEdisk ArraysbyhEtpressingself-assemblednanEspheres CAOyan&CHENyihang(361) OtherOpticalSpectroscopicTechniquesandApplicationsPigment analysis of painted terracotta unearthed from the Western Han Dynasty Tomb at Lianying&Liuji in Yizheng&Jiangsu Province ........................................................................................................................................................................................................FAN Taof e ng(369) Raman Spectrum Analysis of Corrosion Products on the Bronze Bu in Suide Museum in Shaanxi Province...........................................................................................................................FU Qianii,KANG Weidong,ZHANG Shangxin,et al(375) Mechanistic Study of Photocatalytic Overall Water Splitting on NaTaOa-based Photocatalysts by In S iu ATR-IR SpectroscopyDingQian,Ch nTao,F ngZhaochi,tal(311) Rap6dDetect6onofTaxfolnbyL-cyste6neFunct6onalzedCarbon QuantumDotsCHENG Jiawei&ZHANG Yuhui&YANG Jidong(316)。


红外光谱的英文书籍There are several English books on infrared spectroscopy that provide comprehensive and detailed information on the subject. In this article, we will explore some popular books in the field, discussing their content, formatting, and overall presentation. By the end, you will have a better understanding of the available options for studying infrared spectroscopy through English books.1. "Infrared and Raman Spectroscopy: Principles and Spectral Interpretation" by Peter LarkinPeter Larkin's book "Infrared and Raman Spectroscopy: Principles and Spectral Interpretation" is a widely recognized book in the field of vibrational spectroscopy. It covers both infrared and Raman spectroscopy, making it a valuable resource for researchers, students, and professionals.The book is structured logically, starting with the basics and gradually progressing to more advanced topics. It includes extensive explanations of spectroscopic principles, instrumentation, and data interpretation. The chapters are well-organized and utilize clear figures and diagrams to enhance understanding. The author also provides numerous real-world examples and case studies, aiding readers in the practical application of infrared spectroscopy.2. "Introduction to Infrared and Raman Spectroscopy" by N. B. Colthup, L. H. Daly, and S. E. Wiberley"Introduction to Infrared and Raman Spectroscopy" is a classic textbook authored by N. B. Colthup, L. H. Daly, and S. E. Wiberley. It serves as an excellent introduction to the principles of infrared and Raman spectroscopy.The book covers the theory and practice of infrared and Raman spectroscopy, as well as the necessary knowledge of quantum mechanics and group theory. The content is presented in a concise and understandable manner, supported by numerous examples and problems for self-assessment. The authors also provide an extensive range of spectra for reference, allowing readers to compare and identify various functional groups.3. "Infrared Spectroscopy: Fundamentals and Applications" by BarbaraH. StuartBarbara H. Stuart's "Infrared Spectroscopy: Fundamentals and Applications" offers a comprehensive overview of infrared spectroscopy, focusing on its practical applications in various scientific fields.The book begins with an introduction to the basic principles of infrared spectroscopy and instrumentations. It then delves into the applications of infrared spectroscopy in organic and inorganic chemistry, materials science, environmental analysis, and more. Each chapter includes detailed explanations, key concepts, and examples to assist readers in grasping the fundamentals and applying them to real-life scenarios.4. "Infrared Spectroscopy: Theory, Developments and Applications" edited by A. Gauglitz and T. Vo-DinhFor readers interested in more advanced topics, "Infrared Spectroscopy: Theory, Developments and Applications" edited by A. Gauglitz and T. Vo-Dinh is an excellent choice. This book consolidates the latest advancements in infrared spectroscopy, providing a comprehensive reference for researchers and experts in the field.The book covers topics such as Fourier transform infrared spectroscopy, near-infrared spectroscopy, and emerging techniques. It includes contributions from multiple authors, each specializing in different areas of infrared spectroscopy. This collective effort ensures a broad range of perspectives and expertise, making it an invaluable resource for those looking to expand their knowledge in the field.Conclusion:In conclusion, there are several English books available that cover various aspects of infrared spectroscopy. From introductory texts to advanced reference materials, these books provide a wealth of information for individuals interested in the subject. Depending on the specific focus and level of expertise required, researchers, students, and professionals will find one or more of these books suitable for their needs.。

文章编号:100425929(2001)022*******锂离子电池相关材料的Raman 光谱学研究Ξ王兆翔13,李泓1,吴川1,2,高卫东1,3,陈立泉1,莫育俊3,吴锋2,黄学杰1(1中国科学院物理研究所固态离子学实验室,北京 100080;2北京理工大学化工与材料学院,北京 100081;3河南大学物理系,河南开封 475001)摘 要:锂离子电池是目前综合性能最好的可充电池。
本文总结我们实验室用Raman 光谱学研究锂离子电池相关材料的一些结果,包括聚合物电解质的微结构和离子输运机制,低温热解碳负极材料的结构表征和锂离子在其中的嵌入/脱出机理,元素替代引起正极材料LiMn 2O 4的结构变化以及在充放电过程中电极/电解质界面形成的钝化层的性质及其对电池性能的影响。
关键词:Raman 光谱;锂离子电池;电极材料;输运;储存;微结构;钝化层中图法分类号:O657137 文献标识码:R am an Spectroscopic Studies on Materials forLithium Ion B atteriesWAN G Zhao -xiang 13,L I Hong 1,WU Chuan 1,2,G AO Weidong 1,3,CHEN Li -quan 1,MO Yu -jun 3,WU Feng 2,HUAN G Xue -jie 1(1L aboratory f or Soli d S tate Ionics ,Instit ute of Physics ,Chi nese A cadem y ofSciences ,Beiji ng 100080,Chi na ;2School of Chem ical Engi neeri ng and M aterials Science ,Beiji ng Instit ute of Technology ,Beiji ng 100081,Chi na ;3Depart ment of Physics ,Henan U niversity ,Kaif eng 475001,Chi na )Abstract :Lithium ion battery has been believed to be the best among the recharge 2able batteries with its excellent general performances 1We report some of the exper 2imental results of this laboratory by Raman spectroscopy on materials for lithium ion batteries ,including the microstructures and the ion transport mechanism of polymer electrolytes ,the Li +ion storage mechanism in low -temperature pyrolytic carbon anode material ,structural variation induced with elemental substitution in spinel LiMn 2O 4as cathode material for the battery and the characterization of the solid electrolyte interphase (SEI )film on electrolyte/Li -Ag alloy interface in the battery by surface enhanced Raman scattering (SERS )technique 1第13卷 第2期2001年8月光 散 射 学 报CHIN ESE JOURNAL OF L IGHT SCATTERIN G Vol 113 No 12Aug 12001 Ξ收稿日期:200127218 第2期锂离子电池相关材料的Raman光谱学研究2001年K ey w rods:Raman spectroscopy;lithium ion battery;electrode materials;iontransport;Li+ion storage;solid electrolyte interphase(SEI);polymer electrolyte;microstructure1锂离子电池是目前综合性能最好的可充电池,具有工作电压高,能量密度大,循环寿命长,充放电效率高,无记忆效应,自放电率低,对环境无污染等突出的优点,广泛应用于各种便携式电子设备上,并且大有全面取代其它可充电池之势。

第23卷第2期2021年3月宝石和宝石学杂志(中英文)Journal of Gems1GemmologyVol23No2Mar52021中国河南独山玉和菲律宾独山玉中主要矿物的谱学特征王璐,狄敬如(中国地质大学珠宝学院,湖北武汉430074)摘要:对菲律宾吕宋岛独山玉和中国河南独山玉样品进行电子探针、拉曼光谱和红外光谱测试分析。
电子探针结果显示,中国河南独山玉样品中斜长石为连续Na—Ca类质同像系列,而菲律宾独山玉样品中的斜长石主要为钙长石;菲律宾独山玉样品为黝帘石,而中国河南独山玉样品中-黝帘石更多,两个产地样品中含有的透辉石在主要化学成分上一致%拉曼光谱结果显示,中国河南独山玉样品的拉曼吸收光谱为多种长石的混合图谱,而菲律宾独山玉样品中只出现了钙长石的特征吸收峰;两个产地样品中含有的黝帘石和透辉石的拉曼谱峰一致,但其形状、峰值大小和位置都有不同。
红外光谱结果显示,菲律宾独山玉样品中所含长石的红外光谱与标准红外光谱一致,中国河南独山玉样品因类质同象,红外光谱发生改变;中国河南独山玉样品中黝帘石在600〜400cm-1范围的红外光谱谱峰比菲律宾独山玉样品中教量多,且谱峰更加明显;两个产地样品中透辉石在600〜300cm-1范围内的振动频率相同,在1100〜850cm-1范围内的红外光谱略有不同。
通过对其主要矿物y成和谱峰特征的研究,可以为两个产地独山玉的鉴别提供一定的依g%关键词:独山玉;电子探针;拉曼光谱;红外光谱;中国河南;菲律宾中图分类号:TS93文献标识码:A文章编号:20969120(2021)02003808 DOI:10.15964/ki.027jgg.2021.02.005Spectroscopic Study on the Main Mineral of Dushan Yu from Henan Province,China and the PhilippinesWANG Lu#DIJingru(Gemmological Institute,China University of Geosciences,Wuhan430074,China)Abstract:Dushan Yu from Luzon Island of the Philippines and Dushan Yu from Henan Province,Chinawereanalyzedbyelectronprobemicroanalysis,Ramanspectroscopyandin,rared spectroscopy.The EPMA resultsshowedthattheplagioclasein Dushan Yu,rom Henan Province,China,iscontinuous Na-Caisomorphicseries,whiletheplagioclasein Dushan Yu from the Philippines is m ainly anorthite.The Dushan Yu from the Philippine is&-zoisite,whiletheDushanYufrom Chinacontainmore%-zoisite.Themajorchemicalcom-positionsofdiopsideinDushan Yufromthetwooriginsareconsistent.ThetestingresultsshowedthattheRamanabsorptionspectrumofDushanYufrom HenanProvince,China,isa收稿日期:2020-03-08基金项目:中国地质大学(武汉)珠宝检测技术创新中心幵放基金,文章编号CIGTXM-S201727作者简介:王璐(1992—),女,硕士,主要从事宝石学研究工作$通讯作者:狄敬如(1964—),女,副教授,主要从事宝石鉴定与研究工作$E-mail:1016106644@第2期王璐等:中国河南独山玉和菲律宾独山玉中主要矿物的谱学特征39mixture of many kinds of feldspars # while only the characteristic absorption peak of anorthiteappears in Dushan Yu from the Philippines. The Raman peaks of zoisite and diopside in thesamplesfromYheYwooriginsareconsisYenY #howeverYheirshapes #peaksizesandposiYions aredi f erent.ThetestingresultsshowedthattheinfraredspectrumoffeldsparinDushanYufromthePhilippinesisconsistentwiththestandardspectrum #whiletheinfraredspectrumof Dushan Yufrom HenanProvince #China #haschangedduetotheisomorphism.Theinfrared spectrumpeakofzoisiteinDushanYufrom HenanProvince #China #ismoreintherangeof600—400cm —1thanthatinDushanYufromthePhilippines #andthespectrumpeakismore obvious.Thevibrationfrequencyofdiopsideinsamplesfromtwooriginsisthesameintherangeof600—300cm —1#buttheinfraredspectrumisslightlydi f erentintherangeof1100 —850cm —1.Throughtheanalysisofthemainmineralcomponentsandspectralpeakcharac-teristics #acertainbasisfortheidentificationofDushanYufrom HenanProvince #Chinaand thePhilippinescanbeprovided.Key words : Dushan Yu ; EPMA ; Raman spectrum ; infrared spectrum ; Henan Province ,China ; Philippines市场上岀现的产岀于菲律宾吕宋岛的独山 玉和中国河南独山玉有些不同。
㊀㊀收稿日期:2021-01-05基金项目:北京市属高校高水平教师队伍建设支持计划长城学者培养计划(CIT&TCD20190318);北京印刷学院博士启动基金(27170120003/014)第29卷㊀第5期Vol.29㊀No.5北京印刷学院学报Journal of Beijing Institute of Graphic Communication2021年5月May 2021北京一版帆船邮票印刷色料科技分析曹婉颖,刘铮峰,解明思,周文华,施继龙(北京印刷学院印刷与包装工程学院,北京102600)摘㊀要:利用超景深三维视频显微镜㊁激光显微共聚焦拉曼光谱仪㊁扫描电镜-能谱仪对三枚民国时期北京一版帆船邮票印刷色料进行科技分析㊂结果表明,三枚邮票均为手工雕刻凹版印制,灰棕色邮票油墨中的色料是红丹和炭黑,桔黄色邮票为铬酸铅,草绿色邮票为铬酸铅和普鲁士蓝;纸张和油墨中的填料可能是高岭土㊁碳酸钙㊁氧化锌㊁二氧化硅等㊂研究表明,多技术联用在邮票印刷品分析中有较好的应用㊂关键词:北京一版帆船邮票;印刷色料;科技分析中图分类号:TS801文献标志码:A文章编号:1004-8626(2021)05-0155-04㊀㊀帆船邮票是中华民国建立后发行的第一套普通邮票,在其设计上废弃了清代以龙为王权象征的图腾形象,选择了与生活密切相关的帆船,帆船邮票分为三个版别,每一版还设计有农民的劳作形象以及象征国家对教育重视的圜桥牌坊,该邮票的印制㊁发行在中国邮票史上意义重大,具有较高的历史价值和研究价值㊂对于邮票等印刷材料的无损分析方法,前人已有较多成果:甘清等利用显微观测㊁拉曼光谱分析和电镜能谱分析对一张清代绿色蟠龙邮票样品的印刷材料进行无损分析[1];Chaplin 等利用拉曼光谱仪对夏威夷传教士邮票的原版㊁赝品㊁复制品进行了科技分析,还鉴定了早期邮票上的颜料来区分毛里求斯的原始邮票和伪造的邮票[2-3];Widjaja 等利用拉曼光谱识别商业邮票中的染料[4];Badovi-nac 等采用拉曼光谱仪和X 射线光谱仪分析了匈牙利邮票上的颜料[5];裔传臻对拉曼光谱在纸张老化㊁碳黑颜料以及其他颜料上的应用和研究现状进行了梳理归纳[6]㊂本文拟采用激光显微共聚焦拉曼光谱仪㊁扫描电镜-能谱仪和超景深三维视频显微镜等对三枚北京一版帆船邮票油墨中的色料进行科技分析,所得结果还可为邮票等纸制品的保护修复以及科技鉴定提供科技支撑㊂1㊀实验部分1.1㊀样品及测试区域图1为三枚北京一版帆船邮票样品,分别命名为:样品1㊁样品2和样品3,面值为半分㊁壹分和贰分,图幅尺寸20mm ˑ22mm,票幅尺寸22.5mm ˑ24mm,齿孔度数为14度㊂三枚邮票上均有黑色邮戳,无背胶,颜色分别呈灰棕色㊁桔黄色和草绿色㊂该邮票由北京财政部印刷局承印,由外籍雇员海趣依照伦敦版帆船邮票图案雕刻制版印制,于1914年正式发行㊂图1㊀样品及测试区域㊀㊀㊀其中,1-a㊁2-a㊁3-a 分别为三张样品显微测试区域,1-b㊁2-b㊁3-b 为拉曼光谱仪测试区域,3-a㊁3-b㊁3-c 为扫描电镜-能谱仪测试区域㊂1.2㊀实验仪器及工作条件KEYENCE VHX -600超景深三维视频显微镜:放大倍率:ˑ1000㊂Horiba Jobin Yvon XploRA 激光显微共聚焦拉曼光谱仪:常温;暗室;物镜倍率ˑ50,激光器785nm,过滤器50%,孔径100,缝隙200,光栅刻线600gr㊃mm -1,光斑尺寸1μm,曝光时间30s,光谱扫描范围100~3000cm -1㊂JEOL JSM -6610LA 扫描电镜-能谱仪:二次电子信号(SEI);加速电压20kV;测试距离10mm;放大倍率:ˑ30㊂2㊀结果与讨论2.1㊀视频显微镜分析利用KEYENCE VHX -600超景深三维视频显微镜对样品表面的油墨进行显微形貌观察,如图2,由图可知,三个样品的油墨墨层厚实有堆积感且中间部分比边缘部分厚㊁颜色深,图案边缘较清晰,有明显的凹凸感,符合手工雕刻凹版印刷工艺的特点㊂此外,样品1油墨中混有红色颗粒物,样品3中混有桔黄色颗粒物,初步认为这两枚邮票油墨中的色料至少含有两种呈色物质㊂图2㊀测试区域1-a ㊁2-a ㊁3-a 显微形貌图㊀2.2㊀拉曼光谱分析对三张样品的1-b㊁2-b㊁3-b 区域进行拉曼检测,结果如图3~图5,测试结果与参考文献(见表1)进行比对㊂图3㊀测试区域1-b 拉曼光谱图㊀表1㊀参考文献颜料拉曼特征峰颜料拉曼特征峰/cm -1红丹[7]1201522252803133894595477111085炭黑[8]108313801592铬酸铅[9]146336358374401838普鲁士蓝[10]53020982160㊀㊀通过比对可以发现:1-b 的拉曼特征峰与红丹颜料(Pb 3O 4)以及炭黑颜料(C)的特征峰基本吻图4㊀测试区域2-b 拉曼光谱图㊀图5㊀测试区域3-b 拉曼光谱图651北京印刷学院学报2021年合,结合显微形貌图可以初步推断样品1是由桔红色颜料红丹与灰黑色颜料炭黑共同呈色的㊂2-b 拉曼特征峰铬酸铅颜料(PbCrO 4)的特征峰基本吻合,推断样品2的印刷色料是黄色颜料铬酸铅㊂3-b 的拉曼特征峰与铬酸铅颜料(PbCrO 4)以及普鲁士蓝颜料(Fe 4[Fe(CN)6]3)的特征峰基本吻合,结合显微形貌图可以初步推断样品3是由黄色颜料铬酸铅与蓝色颜料普鲁士蓝共同呈色的㊂2.3㊀扫描电镜-能谱仪Mapping 分析对三个样品进行Mapping 分析,结果如图6~图8所示㊂图6㊀测试区域1-c 能谱Mapping 图㊀图7㊀测试区域2-c 能谱Mapping 图㊀样品1:纸张中含有C㊁O㊁Al㊁Si 元素,油墨中含有C㊁O㊁Al㊁Si㊁Ca㊁Fe㊁Zn㊁Pb 元素,且可以明显地看出 1/2 形状㊂C㊁O 元素在纸张中的含量明显高于油墨;Al 元素在纸张中和油墨中含量几乎相同;Si 元素在油墨中的含量略高于纸张;Ca㊁Fe㊁Zn㊁Pb 元素在油墨中的含量明显高于纸张㊂图8㊀测试区域3-c 能谱Mapping 图㊀样品2:纸张中含有C㊁O㊁Al㊁Si 元素,油墨中含有C㊁O㊁Al㊁Si㊁Cr㊁Zn㊁Pb 元素,且可以明显地看出 分 字形状㊂C㊁O 元素在纸张中的含量明显高于油墨;Al㊁Si 元素在油墨中的含量略高于纸张中;Cr㊁Zn㊁Pb 元素在油墨中的含量明显高于纸张㊂样品3:纸张中含有C㊁O㊁Al㊁Si 元素,油墨中含有C㊁O㊁Al㊁Si㊁Cr㊁Fe㊁Zn㊁Pb 元素,且可以明显地看出 贰 字形状㊂C㊁O 元素在纸张中的含量明显高于油墨;Al㊁Si 元素在纸张中和油墨中含量几乎相同;Cr㊁Fe㊁Zn㊁Pb 元素在颜料中的含量明显高于纸张㊂2.4㊀讨论通过分析可知:三张样品中C㊁O 元素主要来源于纸张纤维中的纤维素(C 6H 10O 5)n ,除C㊁O 外的其他元素来源于造纸时添加的胶料㊁填料等辅助材料和油墨中的填料㊁连接料及各种助剂等㊂结合拉曼光谱分析初步确定样品1油墨中的色料为桔红色颜料红丹(Pb 3O 4)和灰黑色颜料炭黑(C);样品2油墨中的色料为黄色颜料铬酸铅(PbCrO 4);样品3油墨中的色料为黄色颜料铬酸铅(PbCrO 4)和蓝色颜料普鲁士蓝(Fe 4[Fe(CN)6]3)㊂两种实验数据可以相互印证和补充,其余检测出的元素推断为纸张和油墨辅助成分可能是高岭土(Al 2O 3㊃2SiO 2㊃2H 2O)㊁碳酸钙(CaCO 3)㊁氧化锌(ZnO)㊁二氧化硅(SiO 2)等物质㊂在印刷色彩学中,青㊁品红㊁黄为色料三原色,不同颜色的油墨在印刷时遵循色料减色法原理,混合色的明度会相对降低㊂样品1呈现灰棕色是由于桔红色主要吸收蓝光和部分绿光,反射红光和部分绿光,灰黑色的加入会使整个混合色明度降低且发黑,一定量混合后最终形成样品1的灰棕色㊂同理,黄色颜料铬酸铅主要吸收蓝光,反射红光和绿光,蓝色颜料普鲁士蓝主要吸收红光,反射蓝光和751第5期曹婉颖,刘铮峰,解明思,等:北京一版帆船邮票印刷色料科技分析绿光,二者通过一定比例的混合就会形成样品3中的草绿色㊂3㊀结论本次实验运用了视频显微镜㊁拉曼光谱仪和扫描电镜-能谱仪分别对三枚1914年北京一版帆船邮票的印刷油墨进行了无损分析㊂测试结果表明:三枚邮票均为手工雕刻凹版印制,样品1灰棕色油墨中的色料是红丹和炭黑,样品2桔黄色邮票油墨中的色料为铬酸铅,样品3草绿色邮票油墨中的色料为铬酸铅和普鲁士蓝㊂根据检测出的元素推测出纸张和油墨中的填料可能是高岭土㊁碳酸钙㊁氧化锌㊁二氧化硅等㊂通过实验研究,将上述三种技术联用能较好地对邮票进行无损分析,进而为邮票等纸制品的保护修复以及科技鉴定提供科技的支撑㊂参考文献:[1]㊀甘清,季金鑫,姚娜,等.清代绿色蟠龙邮票印刷材料无损分析[J].光谱学与光谱分析,2016,36(9):2823-2826. [2]㊀Chaplin T D,Clark R J,Beech D parison of genuine(1851-1852AD)and forged or reproduction Hawaiian Mission-ary stamps using Raman microscopy[J].Journal of Raman Spec-troscopy,2002,33(6):424-428.[3]㊀Chaplin T D,Jurado‐López A,Clark R J,et al.Identificationby Raman microscopy of pigments on early postage stamps:dis-tinction between original1847and1858-1862,forged and repro-duction postage stamps of Mauritius[J].Journal of Raman Spec-troscopy,2004,35(7):600-604.[4]㊀Widjaja E,Garland e of Raman microscopy and band-tar-get entropy minimization analysis to identify dyes in a commercial stamp.Implications for authentication and counterfeit detection [J].Analytical chemistry,2008,80(3):729-733. [5]㊀Badovinac I J,Orlic'N,Lofrumento C,et al.Spectral analysis ofpostage stamps and banknotes from the region of Rijeka in Croatia [J].Nuclear Instruments and Methods in Physics Research Sec-tion A:Accelerators,Spectrometers,Detectors and Associated Equipment,2010,619(1-3):487-490.[6]㊀裔传臻.拉曼光谱在纸质文物研究中的应用[J].文物保护与考古科学,2018,30(3):135-141.[7]㊀Castro K,Rodríguez-Laso M D,Fernandez L A,et al.Fouriertransform Raman spectroscopic study of pigments present in deco-rative wallpapers of the middle nineteenth century from the Santa Isabel factory(Vitoria,Basque Country,Spain)[J].Journal of Raman Spectroscopy,2002,33(1):17-25.[8]㊀闫海涛,孙凯,唐静,等.明代周懿王墓壁画颜料的科技分析[J].华夏考古,2019(2):39-44.[9]Burgio L,Clark R J.Library of FT-Raman spectra of pigments,minerals,pigment media and varnishes,and supplement to exist-ing library of Raman spectra of pigments with visible excitation [J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2001,57(7):1491-1521.[10]E Imperio,G Giancane.Spectral characterization of postagestamps printing inks by means of Raman spectroscopy[J].Ana-lyst,2015,140(5):1702.(责任编辑:周宇)Scientific and Technological Analyses of Printing Pigmentof Beijing First Edition Sailboat StampsCAO Wanying,LIU Zhengfeng,XIE Siming,ZHOU Wenhua,SHI Jilong(Beijing Institute of Graphic Communication,Beijing102600,China) Abstract:Scientific and technological analysis of the printing pigments of three Beijing first edition sailboat stamps during the Republic of China period.These methods include ultra-depth of field three-dimensional video microscope,Raman spectrometer and scanning electron microscope-energy diapersive spectrometer.Results show that all three stamps were printed by hand-engraved intaglio. The coloring substance of the greenish-brown stamp is composed of red lead and carbon black.The orange stamp is lead chromate,and the grass green stamp are lead chromate and Prussian blue.Then, based on the detected elements,it is inferred that the auxiliary components of paper and ink may be kaolin,calcium carbonate,barium sulfate,silicon dioxide and so on.According to the theory of color subtractive method in printing chromatics,the coloring principle of greenish-brown and grass green ink is analyzed.This study have shown that multi-technology has a good application in the analysis of stamp prints.Key words:Beijing first edition sailboat stamp;printing pigment;analysis of science and technology 851北京印刷学院学报2021年。
horiba拉曼光谱光镊技术HORIBA Raman spectroscopy and optical trapping technology refer to the integration of Raman spectroscopy with optical trapping techniques for studying molecules and manipulating micro-objects.Raman spectroscopy is a non-destructive spectroscopic technique that provides information about the molecular composition and structure of a sample by measuring the Raman scattering of light. It is based on the inelastic scattering of photons when they interact with the vibrational modes of the molecules in the sample.Optical trapping, also known as optical tweezers, is a method that uses highly focused laser beams to trap and manipulate microscopic objects, such as nanoparticles, biological cells, or even individual molecules. It enables precise manipulation and measurement of these objects in various scientific and biological applications.HORIBA, a leading manufacturer of scientific instruments, has developed an integrated system that combines Raman spectroscopy and optical trapping technology. This system allows for simultaneous trapping and Raman spectroscopic analysis of micro-objects or molecules.The optical trapping component of the system uses focused laser beams to trap and manipulate the target objects or molecules. By changing the position and intensity of the laser beams, researchers can precisely control and manipulate the trapped objects. This can enable various experiments, such as studying the mechanicalproperties of single molecules or monitoring chemical reactions in real-time.Simultaneously, the Raman spectroscopy component of the system analyzes the trapped objects or molecules. The system collects the Raman scattered light from the trapped sample and analyzes it to obtain detailed information about its molecular composition, structure, and dynamics. This allows researchers to characterize and study the trapped objects or molecules at a molecular level. The integration of Raman spectroscopy and optical trapping technology offers unique advantages in studying and manipulating micro-objects or molecules. It provides precise control and measurement capabilities while allowing for non-destructive analysis. This technology has applications in various fields, including biophysics, single-molecule analysis, materials science, and nanotechnology.。
仪器分析专业名词英文及名词解释仪器分析专业名词英文及名词解释一、紫外-可见光分光光度法1、透光率(transmittance):透过样品的光强度与入射光强度之比。
2、吸收度(absorbance ):透光率的负对数。
3、生色团(chromophore ):含有n—n*或n—n*跃迁的基团。
4、助色团(auxochrome):含孤对电子(非键电子)的杂原子基团。
5、摩尔吸收系数(molar absorptivity):—定波长时,溶液浓度为1mol/L,光程为1cm时的吸收度。
6、比吸收系数(specific absorptivity ):—定波长时,溶液浓度为1%(W/V ),光程为1cm时的吸收度。
7、红移(red shift):化合物结构改变(共轭,引入助色团,溶剂改变等),使吸收峰向长波长移动的现象。
8、蓝移(blue shift):当化合物结构改变或受溶剂的影响等原因使吸收峰向短波长移动的现象。
也称短移(hypso chromic shift )。
9、增色效应(hyperchromic effect):由于化合物结构改变或其他原因使吸收强度增加的效应。
10、减色效应(hypochromic effect):由于化合物结构改变或其他原因使吸收强度减弱的效应。
11、末端吸收(end absorption ):在短波长处(200nm左右) 只呈现强吸收,而不成峰形的部分。
12、标准对照法:在相同条件下配制标准溶液和样品溶液,在选定的波长下分别测定吸光度,根据朗伯-比尔定律计算样品浓度的定量定性分析方法。
13、K带:共轭双键中n—n*跃迁所产生的吸收带,强吸收,£>104。
14、R带:由n—n*引起的吸收带,弱吸收。
15、吸收带:吸收峰在紫外可见光谱中的波带位置(R、K、B和E 带)。
16、B带和E带:芳香族(含芳香族)化合物的特征吸收带。
二、荧光分析法1、荧光(fluorescence):由第一激发单线态的最低振动能级回到基态任一振动能级时发射的光。
肾结石中钙离子含量红外光谱快速分析王静;郝勇【摘要】含钙性肾结石是泌尿结石的主要成分,因此对钙离子含量的快速定量分析具有重要意义.采用衰减全反射傅里叶变换红外光谱法(attenuated total reflection-fourier transform infrared spectroscopy,ATR-FTIR)对由35份肾结石和46份配制结石组成的混合样品集的钙离子进行定量分析,不同的预处理方法和蒙特卡罗变量筛选方法(Monte Carlo uninformation variableelimination,MCUVE)用于模型的优化.光谱经多元散射校正(multiplicative scatter correction,MSC)和MCUVE处理后,模型的交互验证均方根误差(RMSECV)和预测均方根误差(RMSEP)都得到了降低,分别由2.62和3.04减小为2.06和1.88,预测相关系数为0.966.MSC结合MCUVE方法可以提高中红外光谱定量分析钙离子含量的分析精度,为肾结石的成因分析和分类提供一种快速的辅助分析方法.%Calcium in Renal Calculi is the main component for urinary' calculus, therefore, it has important significance for fast determination of the calcium content of renal calculi. Attenuated total reflection-Fourier transform infrared spectroscopy ( ATR-FTIR) technology is adopted for quantitative analysis of the calcium content both in 35 renal calculi and 46 preparation calculi samples. Spectral preprocessing methods and Monte Carlo uninformation variable elimina-tion( MCUVE) are used for model optimization. The RMSECV and RMSEP are reduced from2. 62 and3. 04 to 2. 06 and 1.88,and the correlation coefficient(Rv) is 0.966 when the spectra are preprocessed with multiplicative scatter correction( MSC) and MCUVE methods. MSC combined with MCUVE method can be used for improvingthe analysis precision of the calcium content by using ATR-FTIR technology, it also provide an auxiliary analytical methods for the cause analysis and classification of renal calculi.【期刊名称】《激光与红外》【年(卷),期】2012(042)012【总页数】4页(P1337-1340)【关键词】红外光谱;肾结石;钙离子;小波导数;蒙特卡罗无信息变量消除【作者】王静;郝勇【作者单位】华东交通大学现代教育技术中心,江西南昌330013;华东交通大学机电工程学院,江西南昌330013【正文语种】中文【中图分类】O657.331 引言肾结石是一种世界范围的常见多发病,随着人民生活水平的提高,其发病率也明显提高。
聚氯乙烯变温衰减全反射红外光谱研究于宏伟;吴贤;苏苗苗;关胜琴;孙佳玥;王鑫洁;刘磊;李乐泰;侯腾硕【摘要】采用变温傅里叶变换衰减全反射红外光谱(ATR-FTIR)技术,研究了聚氯乙烯(PVC)的分子结构.实验发现:PVC主要存在着CH2不对称伸缩振动模式(νasCH2)、CH伸缩振动模式(νCH)、CH2对称伸缩振动模式(νsCH2)、CH2弯曲振动模式(δCH2)、CH面内弯曲振动模式(βCH)、CH面外弯曲振动模式(γcH)、CH2面外摇摆振动模式(ωCH2)和C-Cl伸缩振动模式(νC-Cl)等八种红外吸收模式.实验结果发现在303 K-393 K温度范围内,PVC具有良好的热稳定性.研究拓展了变温ATR-FTIR技术在PVC材料热变性方面的研究范围.【期刊名称】《成都纺织高等专科学校学报》【年(卷),期】2017(034)004【总页数】7页(P12-17,21)【关键词】红外光谱;聚氯乙烯;热变性【作者】于宏伟;吴贤;苏苗苗;关胜琴;孙佳玥;王鑫洁;刘磊;李乐泰;侯腾硕【作者单位】石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035;石家庄学院化工学院,河北石家庄050035【正文语种】中文【中图分类】TS102.52+5;O434.3聚氯乙烯(Polyvinyl Chloride 简称 PVC)是氯乙烯单体经加成聚合反应得到的高分子材料[1-2],其分子结构如图 1 所示。
PVC是一类重要的特殊纺织材料[3-4],而热稳定性是影响聚 PVC 加工和使用的重要因素。
PVC 材料性能与其结构密切相关,但常规的红外光谱技术由于制样的困难,很少用于研究 PVC 的分子结构。
Raman spectroscopic study on pressure-induced amorphization innanocrystalline anatase (TiO 2)Zhongwu Wang *,S.K.SaxenaCenter for Study of Matter at extreme conditions (CeSMEC),Florida International University,VH-150,University Park,Miami,FL 33199,USA Received 16November 2000;received in revised form 29January 2001;accepted 30January 2001by F.J.DiSalvoAbstractA raman spectroscopic investigation was carried out to study the pressure-induced phase transformation in nanocrystalline anatase (TiO 2)up to 37GPa.As compared to a macrocrystalline solid which transforms to the a -PbO 2structure at 2.6±4.5GPa,we found that the nano-anatase phase remains stable to pressures as high as ,24GPa,and then transforms to an amorphous phase.The new amorphous phase is quenchable upon release of pressure to ambient condition.An increased surface energy may stabilize the nano-anatase,suppressing the appearance of anatase-to-a -PbO 2phase transformation (2.6±4.5GPa in bulk anatase),and then leads to the amorphization of nano-anatase under strong compression.q 2001Elsevier Science Ltd.All rights reserved.Keywords:A.Nanostructures;D.Phase transition;D.Order±disorder effects;D.Raman spectroscopy PACS :62.50.1p;61.82.Rx;64.70.Kb1.IntroductionNanocrystalline solids are de®ned as materials with crystal size between 10±100nm.They have attracted signi®cant interest due to their special properties [1].Numerous nanocrystalline metals,ceramics and semi-conductors have been synthesized and studied [2,3].Nano-crystalline materials have large surface to volume ratios and fewer dislocations as compared to bulk samples.There is lattice relaxation at grain boundaries.Each of these characters can have signi®cant impact on the physical and electronic properties of the material.Studies have shown that various properties such as stability,hardness,melting temperature,sintering ability,compressibility and electronic structure can be dependent upon particle size [4±9].Nanocrystalline TiO 2has been the subject of considerable experimental and theoretical work in recent years [10±13].The mechanical,optical,electrical behavior,and vibrational modes of nano-TiO 2have been widely studied [10,12,13].In order to better understand the physical and vibrationalproperties of nano-TiO 2,the internal strain and grain boundary structure of nano-TiO 2have been extensively investigated by transmission electron microscopy,X-ray diffraction,and Raman and FTIR spectroscopy [10,11,12,13].But there is little study on the pressure dependence of phase transformations in nano-TiO 2.At ambient pressure,bulk TiO 2crystallizes in three modi-®cations:rutile,brookite and anatase.Because of the simpli-city of synthesis with application in materials science (e.g.nano-techniques)and the relevance to geophysics,high-pressure behavior of bulk anatase has been investigated experimentally by using Raman spectroscopy and X-ray diffraction [14±18].To explore the different dynamics caused by external pressure in nanoparticle materials,we investigated the high-pressure behavior of nano-anatase by using DAC technique together with in-situ Raman spectro-scopy.Here,we report on the pressure-induced amorphiza-tion in nanosized (the average size of 9nm)anatase (TiO 2),and its pressure dependence of Raman vibrational modes.2.ExperimentalThe sample used here was commercial anataseSolid State Communications 118(2001)75±780038-1098/01/$-see front matter q 2001Elsevier ScienceLtd.Allrights reserved.PII:S0038-1098(01)00046-1*Corresponding author.Tel.:11-305-348-3445;fax:11-305-348-3070.E-mail address:zwang04@® (Z.Wang).nanomaterial,with a particle size within 7±11nm.It was identi®ed with X-ray diffraction.At atmospheric pressure,the powder anatase exhibited spectral features similar to these of single crystal [10,19],but the Raman peak in low wave number (141cm 21in bulk-and 158cm 21in nano-anatase)is a little different [12±14],and this is likely caused by heating and plasma effect from laser.High pressure Raman measurements were carried out at room temperature by using a gaseketed high pressure Diamond Anvil Cell (DAC)and Raman spectrometer in the back scattering con®guration.An argon ion laser in combination with an additional Ti 31:sapphire laser was operated at 785nm.This design can effectively suppress the strong ¯uorescence effect of diamond [20].The laser power was operated at 30±50mw (after ®lter)to excite the sample.The Raman spectra were collected by using high throughput holographic imaging spectrograph with volume transmission grating,holographic notch ®lter and thermoelectrically cooled CCD detector with the resolution of 4Rcm 21[20].A 4:1mixture of methanol:ethanol was used as pressure medium.This mixture is known to produce a hydrostatic environment to about 10GPa.Pressures were determined using the pressure dependent spectral shift of the sharp ruby ¯uorescence R 1line [21].The R 1line ¯uorescence was excited by the argon green laser with the wave number of 514.5nm.The sample was placed in a 301steel gasket hole 115m m in initial thickness and 150m m in diameter with a ruby chip.The gasket hole was then completely ®lled with the alcohol mixture to ensure a hydrostatic environ-ment.Moreover,another run without any pressure medium was carried out to check the effect of deviatoric stress on Raman scattering.3.Results and discussionThe Raman spectra of nano-crystalline anatase with a 4:1mixture of methanol/ethanol at high pressures are shown in Fig.1.A signi®cant spectral change occurs at pressure of 24GPa.As is well known,anatase belongs to the spacegroup D 194h (I41/amd)and the primitive unit cell contains two formula units [14].According to the factor group analy-sis,the six modes (A 1g 12B 1g 13E g )are Raman active and the three modes (A 1u 12E u )infrared active [14].At low pressure,all Raman active modes are in reasonable agree-ment with these reported previously (Fig.1)[10,13].Because of the plasma effect from Ti 31:sapphire laser and heating in sample,the lowest wave number mode (E g )in 162cm 21is a little higher than that (158cm 21)observed previously at ambient condition by using an argon ion laser of 488nm [10,13],and also shows a relatively weak peak.Our studies on other powders including garnets and oxides show a similar phenomenon,which can be effectively suppressed under high pressures [20].This can also be found in Fig.1,showing that the above Raman peak inlow wave-number mode (E g )gets stronger with increasing pressure.As shown in Fig.2,the four strong Raman peaks,assigned to two E g modes and one B 1g mode and one A 1g 1B 1g duplicated mode,shift to higher frequencies under compression.The three high wave-number modes have similar Raman shift slope of 2.7cm 21/GPa,similar to these observed in bulk materials [17].One low wave number mode (E g )shows a lower slope of 1.5cm 21/GPa,much lower than that in bulk materials [17].With increasing pressure to about 24GPa,all Raman modes disappear.In stead,a halo starts to arise and remains up to our investi-gated pressure of 37GPa (Fig.1).It means that a pressure-induced amorphization occurs at pressure of about 24GPa.Upon release of pressure,the amorphous phase was quench-able to ambient pressure.In the run of free pressure medium,the spectral depen-dence of pressure is the almost same as that with pressure medium,and the amorphization occurs at same pressure of 24GPa.Also,a few ruby chips in the loading sample were used to examine the pressure gradient,indicating that no remarkable pressure gradient cross the sample exists at pressure lower than 10GPa.This implies that the nano-crystalline anatase may have a low bulk modulus as compared with the bulk anatase (179GPa)[15].Z.Wang,S.K.Saxena /Solid State Communications 118(2001)75±7876Fig.1.High pressure Raman spectra of nano-anatase at room temperature.The spectra were collected from sample with a pres-sure medium of a 4:1mixture of methanol/ethanol.The run without pressure medium has the same spectral dependence on pressure.For the bulk crystalline anatase,both Raman and X-ray diffraction studies indicate that anatase transforms to the orthorhombic columbite phase(a-PbO2structure with space group Pbcn,60)at2.6±4.5GPa,and then to the monoclinic baddeleyite phase(ZrO2structure with space group P21/c,14)at13GPa[14±19].Upon decompression, the baddeleyite phase transforms to the a-PbO2phase at about7GPa[16].As compared to the bulk materials, nano-anatase has a higher ratio of surface energy to volume and a ratio of lower defect number to volume[2,3].Also, nano-crystalline anatase may have different physical proper-ties,likely having a lower bulk modulus.Thus,a high energy kinetic hindrance may exists in the phase trans-formation of anatase-to-a-PbO2due to the size-induced effect,so it is likely to result in anatase stable up to very high pressure(24GPa),and then in turn to transform to an amorphous phase,rather than a new a-PbO2phase or ZrO2 phase at high pressure.4.ConclusionAn in-situ Raman spectroscopic study of nanocrystalline anatase with an average size of9nm has been conducted up to37GPa.It is shown that the pressure-induced amorphiza-tion in nano-anatase takes place at pressures around24GPa. The decrease of the particle size results in an anatase phase stable up to very high pressure,and suppresses the appear-ance of the anatase-a-PbO2phase transformation observed in the corresponding bulk material.It is suggested that a higher ratio of surface energy to volume and a ratio of defect number to volume as well as the size-induced effect in nano-anatase compared with the bulk material may stabilize TiO2 in the original low pressure anatase phase,and then lead to the amorphization of nano-anatase under strong compres-sion.AcknowledgementsWe thank Florida International University for the research was made possible through®nancial support from the Division of sponsored Research at FIU.We also thank Peter Lazor for invaluable discussion,Debby Arnold for editorial and Peter Liermann for technical assistance.References[1]H.Gleiter,Prog.Mater.Sci.33(1989)223±315.[2]R.W.Siegel,Mater.Sci.Engng.A168(1993)189±197.[3]A.P.Alivisatos,P.F.Barbara,A.W.Castleman,J.Chang,D.A.Dixon,M.L.Klein,G.L.McLendon,ler,M.A.Ratner, P.J.Rossky,S.I.Stupp,M.E.Thompson,Adv.Mater.10 (1998)1297±1336.[4]C.-C.Chen,A.B.Herhold,C.S.Johnson,A.P.Alivisatos,Science276(1997)398±401.[5]S.H.Tolbert,A.B.Herhold,L.E.Brus,A.P.Alivisatos,Phys.Rev.Lett.76(1996)4384±4387.[6]S.H.Tolbert,A.P.Alivisatos,J.Chem.Phys.102(1995)4642±4671.[7]S.B.Qadri,J.Yang,B.R.Ratna,E.F.Skelton,J.Z.Hu,Appl.Phys.Lett.69(1996)2205±2207.[8]J.Z.Jiang,L.Gerward,R.Secco,R.Frost,J.Appl.Phys.87(2000)2658±2660.Z.Wang,S.K.Saxena/Solid State Communications118(2001)75±7877Fig.2.Pressure dependence of the peak frequencies of the Raman active modes of anatase up to24GPa at room temperature.[9]A.N.Goldstein,C.M.Echer,A.P.Alivisatos,Science256(1992)l425±1428.[10]W.F.Zhang,Y.L.He,M.S.Zhang,Z.Yin,Q.Chen,J.Phys.D.Appl.Phys.33(2000)912±916.[11]S.D.Mo,W.Y.Ching,Phys.Rev.B.51(1995)13023±13032.[12]D.Bersani,P.P.Lottici,X.Z.Ding,Appl.Phys.Lett.72(1998)73±75.[13]J.C.Parker,R.W.Siegel,Appl.Phys.Lett.57(1990)943±945.[14]M.Nicol,M.Y.Fong,J.Chem.Phys.54(1971)3167.[15]T.Arlt,M.Bermejo,M.A.Blanco,L.Gerward,J.Z.Jiang,J.S.Olsen,J.M.Recio,Phys.Rev.B61(2000)14414±14419.[16]J.K.Dewhurst,J.E.Lowther,Phys.Rev.B54(1996)R3673±R3676.[17]T.Ohsaka,S.Yamaoka,O.Shimomura,Solid State Commun.30(1979)345±347.[18]J.F.Mammone,S.K.Sharma,M.Nicol,Solid State Commun.34(1980)799±802.[19]T.Ohsaka,F.Izumi,Y.Fujiki,J.Raman Spectrosc.7(1978)321.[20]O.N.Shebanova,zor,Z.Wang,submitted for publica-tion.[21]H.K.Mao,P.W.Bell,J.W.Shaner,D.J.Steinberg,J.Appl.Phys.49(1978)3276±3284.Z.Wang,S.K.Saxena/Solid State Communications118(2001)75±78 78。