FDA 对三聚氰胺安全风险评估
- 格式:doc
- 大小:68.50 KB
- 文档页数:9

HPLC法测定奶粉中三聚氰胺含量的不确定度评估1.目标为了三聚氰胺测量方法的优化和更准确地评价三聚氰胺的结果,采用B类评定方式确定该方法的相对不确定度。
2.测定程序2.1.方法依据:GB/T 2388-2008《原料乳与乳制品中三聚氰安检测方法》2.2.样品提取称取3g(精确至0.01g)试样于50mL具塞塑料离心管,加入15mL三氯乙酸溶液和5mL 乙腈,超声提取10min,再振荡提取10min后,以不低于4000r/min离心10min。
上清液经三氯乙酸溶液润湿的滤纸过滤后,用三氯乙酸溶液定容至25mL,移取5mL滤液,加入5mL水混匀后做待测净化液。
2.3.净化将待净化液转移至固相萃取柱(BESEP HR-XC)中。
依次用3mL水和3mL甲醇洗涤,抽至近干后,用6mL氨化甲醇溶液洗脱。
整个固相萃取过程流速不超过1mL/min。
洗脱液于50℃下用氮气吹干,残留物用1mL流动相定容,涡旋混合1min,过微孔滤膜后,供HPLC测定。
2.4.液相色谱条件a)C18柱,250m m×4.6mm×5umb)离子对试剂缓冲溶液-乙腈(88+12,体积比)。
c)流速:1.0mL/mind)柱温:40℃e)波长:240nmf)进样量:20uL2.5.标准曲线的绘制2.5.1.标准储备液的配制准确称取0.2330g三聚氰胺标准品于50mL容量瓶中,用甲醇水溶液溶解并定容至刻度,配制成浓度为464ug/mL的三聚氰胺标准储备液。
2.5.2.标准工作液用流动相将三聚氰胺标准储备液逐级稀释得到浓度分别为0.8、2、20、40、80ug/mL的标准工作曲线,浓度由低到高进样检测,以峰面积-浓度做标准曲线,得到标准曲线回归方程。
具体配制过程见下表:表1 标准工作液配制过程标准工作液浓度(ug/mL )稀释过程吸取液浓度(ug/mL ) 吸取量(mL )定容体积(mL )80 464 1.725 10 40 80 5 10 20 80 2.5 10 2 80 0.25 10 0.8800.1103. 建立数学模型试样中三聚氰胺结果计算公式如下:fm V C X ⨯⨯⨯⨯=10001000式中:X ——试样中三聚氰胺的含量,单位是毫克每千克(mg/kg ); C ——样液在标准曲线所读的浓度值; V ——样液最终定容体积,单位为mL ; m ——试样的质量,单位为克(g ); f ——稀释倍数。

三聚氰胺的毒理学研究进展三聚氰胺(Melamine)又名蜜胺,氰尿三酰胺,三聚氰酰胺,由德国化学家Justus von Liebig 于1834年首次合成,是一种用途广泛的基本有机化工中间产品,目前最主要的用途是作为生产三聚氰胺/甲醛树脂(MF)的原料,普遍应用于建材、灭火剂、纺织、皮革、造纸等行业,也常作为生产塑料食品包装材料的单体和助剂,还可以作为合成药物的中间体或药物载体的原料。
但三聚氰胺本身既不是药品也不是食品添加剂,仅仅是一种化工原料,正常情况下不应该出现在食品或动物饲料中。
2008年9月8日在我国甘肃省陆续报道多例婴幼儿泌尿系统结石病例,随后多个省份相继发现类似病例,调查发现此类患儿多有食用三鹿牌婴幼儿配方奶粉的历史。
据卫生部报告,截至2008年9月17日8时,全国医疗机构共接诊、筛查曾食用过三鹿牌婴幼儿配方奶粉的婴幼儿数万名,临床确诊泌尿系统结石患儿6244名(回顾性调查发现4例死亡)。
9月16日国家质量监督检验检疫总局通报全国婴幼儿奶粉三聚氰胺含量专项检查结果显示,有22家企业69批次产品中检出了含量不同的三聚氰胺,其中石家庄三鹿牌婴幼儿配方奶粉中含量较高,最高含量达2563mg/kg,其它抽检奶粉中三聚氰胺最高含量在0.09-619mg/kg之间;9月18日国家质量监督检验检疫总局通报全国液态奶三聚氰胺专项检查结果显示,市场上绝大部分液态奶是安全的,除蒙牛、伊利、光明部分批次产品中检出三聚氰胺含量在0.6-8.6mg/kg之间外,其余被检查的406家企业生产的847批次的液态奶产品未检出三聚氰胺。
2007年3月16日,因美国发生4000多起猫、狗等宠物食品中毒引发肾脏损害的事件,加拿大Menu食品公司生产的6亿多个包装的宠物食品被美国食品药品管理局(US FDA)紧急召回,调查确认此次事件与部分从中国进口的动物饲料原料——小麦蛋白粉和大米蛋白粉中三聚氰胺的污染有关。
2004年3月,在亚洲韩国和中国台湾曾发生6000多起狗和少数猫因进食某种品牌宠物饲料而导致肾功能衰竭的事件,此次事件中中毒动物的临床症状、组织病理学检查和毒性表现与2007年北美宠物饲料事件如出一辙,已有多项实验研究支持这两次宠物饲料中毒事件有共同的起因——三聚氰胺污染。

中国爆发了三聚氰胺危机,食品安全事关无数人的生命和健康,反映激烈,反响巨大也属正常。
事件发生后,一些人的愤怒习惯性地必然超出就事论事,将矛头直接指向中国质量检测机制,以及中国人所谓丑陋的劣根性。
在某种很坏的思维习惯影响下,“中国如何,外国如何”之类的比较又借机喧嚣一时,结论无非是中国糟得很,外国好得很,一时间,消费者纷纷放弃购买国产奶制品,涌向国外产品货架。
事发之后,中国政府有关部门采取了一系列措施应对三聚氰胺危机,有关当事人的最终处理还没有结果,但是,在国产奶制品中消除三聚氰胺潜在危害的工作一项项地进行着。
其中最重要的措施之一是,制定了奶制品中三聚氰胺的最低检出量标准。
即便如此,一些人还是不满意。
比方说,美国食品和药物管理局(FDA)就大唱高调,他们摆出一副高度重视人权和生命的姿态说:对于婴儿,不存在所谓安全水平的三聚氰胺。
也就是说,美国人大约在一个月前气冲霄汉地宣称,奶制品中应该没有丝毫三聚氰胺的存在。
这种表现,使得一些跟在美国屁股后面的人,再次感受到美国的伟大,中国的腐朽。
他们诋毁中国的习惯,又得到了现实事例的支持;中国黑暗崇拜者又得到了物质和精神上的滋养。
曾经指出过三聚氰胺问题的根本,不在于事先检测,而在于工业化、商业化本身,以及一切为金钱、放弃道德的社会流风,结果遭人围攻,说是为政府辩护。
对于某些人来说,中国社会发生任何不好的事情,不追究政府责任就是粉饰太平和稀泥;只有攻击政府,才是常态性的正确;凡是只要把矛头指向政府,即便不对也对了七分,也就能获得无知者的喝彩;只有攻击政府才是真正的为民请命,才是道德责任感的体现;如果再援引西方如何、美国如何来攻击中国政府,那就更加是绝对正确了,更加立于不败之地了。
攻击中国政府,攻击中国体制,攻击全体中国人,已经成为某些人批判社会现实的唯一常规战术,无需深入思考,贴上这个标签便能煞有介事地招摇过市。
日前,美联社报道,在美国销售的雀巢、美赞臣、雅培三种品牌,在其婴儿产品中,也检出了检测方法是美国FDA提供的方法,至于这几种品牌在中国销售的产品是否也有三聚氰胺,其中国地区负责人竭力声明,在中国的产品是安全的,因为已经按照中国政府制定的标准检测了。

三聚氰胺新标准引争议7月6日,联合国负责食品安全标准的机构国际食品法典委员会设立了一项新标准,对食品中的三聚氰胺含量做出了规定。
这似乎让所有消费者震惊,难道食品中出现三聚氰胺是“合法”?关于各国对于三聚氰胺的妥协,也是颇有一番趣味。
虽然明知三聚氰胺是化工原料,有毒,且不能食用。
但任何一个国家面对它却如同斗败的公鸡,不得不向它低头。
这究竟是利益在作祟,还是其他原因?A掀开盖头在2008年以前,中国消费者没有多少人知道三聚氰胺是什么,但当2008年那场轰动全国乃至全世界的“三鹿”事件,让“三聚氰胺”家喻户晓。
在奶粉中出现三聚氰胺,意在提高蛋白含量,但三聚氰胺有毒,导致出现众多“结石宝宝”,这不仅是“三鹿”的死穴,国家在大力排查“三鹿”的同时,也同样把视线转向所有奶粉行业,一时间,奶企都被三聚氰胺的噩梦缠绕着。
但让人无奈的是,由于三聚氰胺并未纳入奶粉检测标准中,国家对三聚氰胺的排查有些举步艰难,在这样的情况下,2008年10月7日,由卫生部、工业和信息化部、农业部、工商总局、质检总局联合印发了关于乳制品及含乳食品中三聚氰胺临时管理限量值规定的公告。
公告中规定,婴幼儿配方乳粉中三聚氰胺的限量值为1mg/kg,高于1mg/kg的产品一律不得销售。
液态奶(包括原料乳)、奶粉、其他配方乳粉中三聚氰胺的限量值为2.5mg/kg,高于2.5mg/kg的产品一律不得销售。
含乳15%以上的其他食品中三聚氰胺的限量值为2.5mg/kg。
规定出台后,职能部门对奶粉内三聚氰胺的排查更加有力,也有理。
但这却让我们忽略了公告中的一个词:临时。
B美国人的妥协我国出现“三聚氰胺奶粉”,最初美国是嘲笑的,嗤之以鼻。
2008年10月,即中国奶粉污染事件之后,FDA(美国食品和药物管理局)曾在一份公告中传达了其对于三聚氰胺的“零容忍”政策,即“目前,婴儿奶粉中任何含量的三聚氰胺以及与三聚氰胺有关的化学物质都无法确认为对婴儿安全”。
言外之意,婴儿奶粉中“不得检出”三聚氰胺,只要出现三聚氰胺就是不安全的。

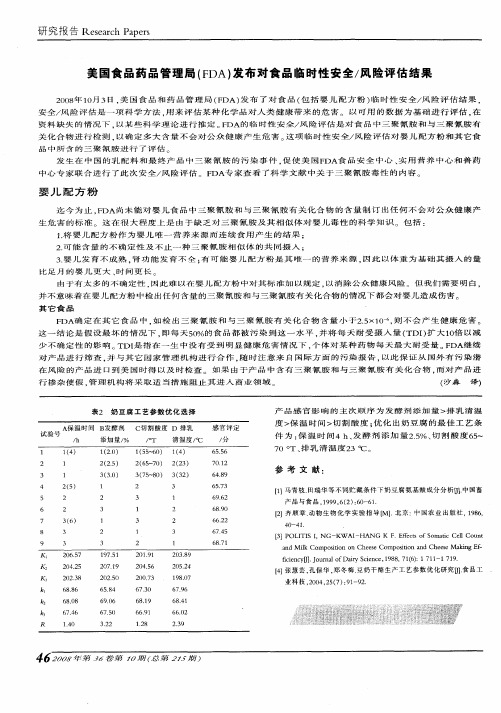
三聚氰胺毒理学安全性评估张晓鹏李宁(中国疾病预防控制中心营养与食品安全所,北京,100050)摘要: 对三聚氰胺的毒理学安全性资料包括代谢、急性毒性、遗传毒性、亚慢性毒性、慢性毒性和致癌性、人群可能暴露水平、危险性进行评估进行了综述。
关键词:三聚氰胺;毒理学;危险性评估由于“三鹿奶粉”事件和“宠物饲料”事件,三聚氰胺的毒理学安全性倍受关注。
三聚氰胺是一种重要的氮杂环有机化工原料,主要用于生产三聚氰胺-甲醛树脂,广泛用于木材加工、塑料、涂料、粘合剂、造纸、纺织、皮革、电气、医药等行业,目前是重要的尿素后加工产品。
此外三聚氰胺还可以用于阻燃剂、防水剂、防皱剂、甲醛清洁剂等,本文对三聚氰胺安全性毒理学资料包括代谢、急性毒性、遗传毒性、亚慢性毒性、慢性毒性和致癌性、人群可能暴露水平、危险性进行了综述,。
1. 理化性质三聚氰胺[1](CAS No.108-78-1),英文名melamine,别名2,4,6-三氨基均三嗪(2,4,6-triamino symtriazine);分子式C3H6N6,分子量126.12,为白色、单斜晶体;密度为1574 kg/m3,熔点350℃,蒸汽压4.7 x 10-8 Pa (20℃);它在水中的溶解度为3.1 g/L (20℃),微溶于乙二醇、甘油、乙醇,不溶于乙醚、苯;280℃以上发生分解,不易燃易爆;无生物蓄积性,易降解。
2. 代谢三聚氰胺在大鼠体内不参与任何形式的代谢或者说呈惰性状态。
14C标记的三聚氰胺进入Fischer 344大鼠体内后主要分布于血液、肝脏、肾和膀胱等器官,只有肾和膀胱中14C浓度高于血浆,膀胱中最高;血浆中三聚氰胺的半衰期为2.7h,尿液排出三聚氰胺的半衰期为3h,24h内几乎全部从尿液排出,组织内也无残留;呼吸和粪便未检测到14C[2]。
3. 毒性3.1环境生态毒性:3.1.1急性毒性鱼[3]:高体雅罗鱼48小时半数致死浓度(LC50,48h)> 500 mg/L;青鳉鱼LC50,48h = 1000 mg/L;孔雀鱼96小时半数致死浓度(LC50,96h)> 3000 mg/L;孔雀鱼96小时10%致死浓度(LC10,96h)> 4400 mg/L。

三聚氰胺的风险和危害以及检测方法三聚氰胺是一种有机化合物,也称为三聚氰酸或三氰胺。
它是一种白色晶体,具有熔点和化学性质不稳定的特点。
三聚氰胺主要应用于有机合成和化工生产中,例如用于生产甲醛、涂料、粘合剂和皮革等。
一、三聚氰胺的基本概念三聚氰胺是一种天然产品,早已在自然中发现,也可以通过化学合成的方法获得。
它是一种重要的有机化合物,在工业和日常生活中有着广泛的应用。
1. 三聚氰胺的结构与性质三聚氰胺分子式为C3N3(NH2)3,结构中有三个氮原子和六个氢原子,氮原子和氢原子的比例为1:3,与蛋白质中氮氢比相同。
三聚氰胺的结构中含有三个氨基(NH2)基团,它可以与羧基(COOH)基团发生反应,生成稳定的酰胺键(COO)。
2. 三聚氰胺的制备方法三聚氰胺的制备方法主要有两种:一是通过尿素或甲酰胺高温分解得到;二是通过丙烯腈或乙撑亚胺反应得到。
3. 三聚氰胺的应用领域三聚氰胺在工业和日常生活中有着广泛的应用。
主要应用领域包括:(1)甲醛生产:三聚氰胺可以用于生产甲醛,甲醛进一步反应可以生产出各种有机化合物,如醇类、酚类、酯类等。
(2)涂料:三聚氰胺与其他化合物反应可以生产出各种涂料,如醇酸树脂涂料、丙烯酸树脂涂料等。
(4)粘合剂:三聚氰胺可以用于生产各种粘合剂,如环氧树脂粘合剂、聚氨酯粘合剂等。
(5)皮革:三聚氰胺可以用于鞣制皮革,可以使皮革变得柔软、耐磨。
二、三聚氰胺的风险与危害三聚氰胺虽然在工业和日常生活中有着广泛的应用,但是它也是一种有毒的化合物。
以下是一些可能引起的健康问题:1. 过敏反应:有些人对三聚氰胺过敏,会出现皮肤瘙痒、皮疹等症状。
2. 眼部刺激:三聚氰胺对眼睛有一定的刺激作用,会引起流泪、疼痛等症状。
3. 毒性作用:三聚氰胺是一种有毒的化合物,对肝脏、肾脏、神经系统等都有一定的毒性作用。
4. 致癌性:三聚氰胺已经被证明具有致癌性,可以导致膀胱癌、肝癌等疾病的发生。
三、三聚氰胺在环境中的影响三聚氰胺在环境中主要通过降雨、降雪和径流等途径进入水体,也可以通过挥发和吸附等途径进入土壤和大气。

毒奶粉的危害
小编希望毒奶粉的危害这篇文章对您有所帮助,如有必要请您下载收藏以便备查,接下来我们继续阅读。
本文概述:最近曝光一些毒奶粉,为了利益,一些不法商在奶粉里添加三聚氰胺。
那么毒奶粉的危害是什么呢?下面和小编会给您答案。
背离原本奶粉应有的价值,制造商为低价谋利,从中添加不宜小儿生长发育的不利营养素,造成小儿不同程度的伤害,众人俗称之为毒奶粉。
那么毒奶粉的危害是什么呢?下面和小编了解下毒奶粉对幼儿有哪些危害。
2008年6月中旬后,三鹿又陆续接到婴幼儿患肾结石等病状去医院治疗的信息。
南方日报收到网民反应,有人在国家质量监督检验检疫总局食品生产监管司的留言系统里反映由三鹿奶粉导致多起婴儿肾结石,但事后屏蔽,只有要求网民提供详情的留言回复尚在。
7月,徐州儿童医院小儿泌尿外科医生冯东川在国家质检总局食品生产监管司的留言系统里反映今年婴儿双肾结石导致肾衰的病例出奇地增多,且大多饮用三鹿奶粉,并表示希望政府部门能组织流行病学专家协助明确原因,不过也是没有得到明确答复。
实验表明,三聚氰胺的毒性较低,在动物体内代谢很快而且不会残留在体内,主要对膀胱和肾脏有影响,引发动物膀胱炎、膀胱结石、肾脏炎症等,但没有发现。



/~dms/melamra4.htmlInfants may be more sensitive than adults to exposures because, for example, infant formula is the sole source of nutrition, exposure continues for up to 12 months, and renal function may be more immature compared to adults. This raises a high degree of uncertainty with regard to the determination of safety/risk. Given these conditions, FDA has applied an additional 10-fold safety factor, yielding a combined safety factor of 1000-fold, to compensate for these uncertainties. This results in a TDI/10 of 0.063 mg melamine/kg-bw/d.The next step is to convert from a dose of 0.063 mg/kg bw/d to total melamine consumed per day.0.063 mg/kg-bw/d x 3 kg/infant = 0.189 mg melamine/infant/day.To estimate the level of melamine that does not raise public health concerns, FDA used a worst case exposure scenario in which all of an infant’s total daily dietary intake (typically 0.15 kg powdered infant formula) is contaminated with melamine. The previously determined (see above) total amount of melamine/infant/day:0.189 mg/infant/day divided by 0.15 kg of food = the food contamination level that would provide this amount of melamine to a 3 kg infant per day. Thus, 0.189 mg melamine divided by 0.15 kg of food = 1.26 mg melamine/kg food.Therefore, if 100% of the diet were contaminated at a level of 1.26 ppm of melamine, an infant’s daily intake would equal 0.063 mg/kg bw/d. This value of 1.26 ppm is rounded down to 1.0 ppm melamine to provide an additional margin of safety.The safety/risk assessment assumes the analogues to have equal effect. Thus, levels of melamine or one of its analogues alone below 1.0 ppm in infant formula do not raise public health concerns.FDA is continuing to sponsor and conduct animal studies to assess the potential toxicity from co-ingestion of melamine and cyanuric acid. As this ongoing research is completed, FDA will updateits interim safety/risk assessment for melamine and its analogues, as appropriate.。

环境风险评价概论学院:环境与化学工程学院班级: 11级环境科学02班学号: 41104060231姓名:刘倩三聚氰胺的危害1.三聚氰胺对人体的毒性由于三聚氰胺为常见的化工产品,故以前多作为职业暴露进行分析。
工业生产中工人可能吸入或皮肤接触,但对健康的危害不大。
三聚氰胺会随着工业生产废弃物进入水、土壤和大气中,并有少量通过空气或食物进入人体。
由于三聚氰胺可用于生产食品包装材料、农药和化肥,因此食品中可能会有微量的三聚氰胺。
此外,采用三聚氰胺-甲醛树脂制作的食品餐具在与食品接触时会有微量的三聚氰胺迁移出来。
此前并无明确的三聚氰胺毒性的人群资料,但一般认为不会产生永久性损伤或死亡。
经实验显示,三聚氰胺在体内不会被分解,主要经尿液排出体外。
虽然三聚氰胺能给实验动物造成泌尿系统的肿瘤,但并没有足够的证据证明它对人体有致癌性。
因此IARC将三聚氰胺定为Ⅲ级致癌物,对于此次三聚氰胺中毒事件,卫生部的统计数据表明,超过99%的患者是3岁以下儿童。
有关专家分析,我们可以了解到,长期、大量口服三聚氰胺可致婴儿泌尿系结石。
2008年10月3日美国食品和药物管理局再次对三聚氰胺的食用安全性进行了评价,结果认为,除婴儿配方奶粉外,三聚氰胺含量在2.5mg/kg以下的食物对人体是安全的。
由于婴儿配方奶粉是婴儿的主要食物,相对于成年人来说,婴儿可能通过膳食摄入的三聚氰胺量远远高于成年人,因此三聚氰胺对于婴儿的危害高于成人。
2.三聚氰胺对动物的毒性三聚氰胺对哺乳动物低毒:大鼠连续 2 h 吸入三聚氰胺粉尘200 mg/m3,未见中毒症状。
大鼠吸入三聚氰胺粉尘100~380 mg/m3,2次/天,6 次/周,连续4个月以上,出现体重增加迟滞、中枢神经系统及肾功能紊乱、肺内炎性改变等,长时间反复接触可对肾脏造成损伤,对眼及皮肤无刺激作用。
三聚氰胺的急性毒性:三聚氰胺对老鼠的半数致死量为3 248 mg/kg,对兔子的半数致死量为每千克 1 000 mg/kg。
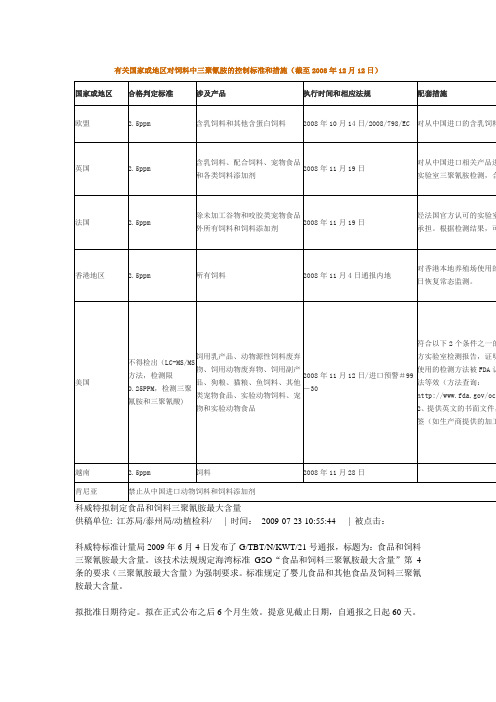
有关国家或地区对饲料中三聚氰胺的控制标准和措施(截至2008年12月12日)
科威特拟制定食品和饲料三聚氰胺最大含量
供稿单位: 江苏局/泰州局/动植检科/ | 时间:2009-07-23 10:55:44 | 被点击:
科威特标准计量局2009年6月4日发布了G/TBT/N/KWT/21号通报,标题为:食品和饲料三聚氰胺最大含量。
该技术法规规定海湾标准GSO“食品和饲料三聚氰胺最大含量”第4条的要求(三聚氰胺最大含量)为强制要求。
标准规定了婴儿食品和其他食品及饲料三聚氰胺最大含量。
拟批准日期待定。
拟在正式公布之后6个月生效。
提意见截止日期,自通报之日起60天。
智利对进口中国甜橙等枝芽制定植物卫生要求
智利近日发出G/SPS/N/CHL/300号通报,智利农畜局(SAG)针对进口中国甜橙(Citrus sinensis)及蜜橘(Cit?鄄rus reticulata)繁殖材料制定植物卫生要求。
主要内容是规定了智利进口此类材料的植物卫生要求与管理程序。
该卫生要求的拟生效日期为2009年7月30日。

FDA敦促对药品进行三聚氰胺污染检验
无
【期刊名称】《国外药讯》
【年(卷),期】2009(000)010
【摘要】美国FDA发布一个高危三聚氰胺污染药物成分用于生产成品前进行三聚氰胺检验的指南。
2007年源自中国的宠物食品成分及2008年中国婴儿配方三聚
氰胺污染事件促成了本指南的出台。
本指南适用于成品药物生产企业、分包装企业、其他供应商及配药药剂师。
【总页数】2页(P4-5)
【作者】无
【作者单位】无
【正文语种】中文
【中图分类】R95
【相关文献】
1.FDA药品安全通讯:FDA要求对某些非处方外用消毒产品进行标签变更和一次
性包装以降低感染风险 [J],
2.美国FDA要求对用于睡眠障碍治疗的所有药品说明书进行更改 [J], 马坤;王庆
利
3.01004 敦促FDA制订已有药品/器械组合产品更好的指南 [J], 郑晶心
4.美国FDA加强对养殖水产品药品残留量的检验 [J],
5.FDA着手进行非专利生物技术药品等同_陛判断 [J], 谷本佐理名
因版权原因,仅展示原文概要,查看原文内容请购买。

美发布三聚氰胺标准2.5ppm以下不会对健康有害
佚名
【期刊名称】《生命科学仪器》
【年(卷),期】2008(6)10
【摘要】被境外卫生机构指检出三聚氰胺的食品名单越来越多,让购买了这些产品的市民十分为难。
到底食物是否绝对不含有三聚氰胺才能安全食用呢?虽然各国对三聚氰胺检测标准不一,不过美国食品药品监督管理局(FDA)上周五发布的标准则表示,三聚氰胺含量2.5ppm以下(即2.5毫克/公斤)不会对公众健康产生危险。
据了解,我同国家标准委也正针对三聚氰胺组织有关专家修订乳品相关标准。
【总页数】1页(P16-16)
【关键词】三聚氰胺;检测标准;公众健康;食品药品监督管理局;美发;国家标准委;卫生机构;安全食用
【正文语种】中文
【中图分类】TS201.6;TQ226.62
【相关文献】
1.国家质检总局、国家标准委发布《原料乳与乳制品中三聚氰胺检测方法》国家标准 [J],
2.国家质检总局、国家标准委发布《原料乳与乳制品中三聚氰胺检测方法》国家标准 [J], 无
3.国家质检总局、国家标准委批准发布《原料乳中三聚氰胺快速检测液相色谱法》
国家标准 [J],
4.卫生部:乳品安全标准中不设置三聚氰胺相关规定 [J],
5.国家质检总局、国家标准委批准发布《原料乳中三聚氰胺快速检测液相色谱法》国家标准 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
See November 28, 2008 UpdateInterim Safety and Risk AssessmentofMelamine and its Analogues in Foodfor Humans aBACKGROUNDOn September 11, 2008, FDA learned that melamine may be contained in an infant formula manufactured by a firm in China. As of September 21, 2008, FDA learned that a total of 52,857 cases of nephrolithiasis (and, in some instances, renal failure) had been reported in China linked to consumption of this contaminated powdered formula. There have been approximately 13,000 hospitalizations, and at least 3 deaths have been confirmed to date. The vast majority of illnesses involved children under the age of 3 years (82% < 2 years; 17% 2-3 years; 0.8% > 3 years; and no cases involved adults). The results of an investigation conducted in China indicated that Chinese-produced powdered infant formula was linked to these illnesses; no cases were associated with liquid infant formula. An investigation of powdered formulas was conducted nationally by China's General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) and revealed contamination of powdered formulas produced by 22 companies. Test results conducted in China on samples of the powdered infant formula showed that they contained a wide range of concentrations (0.1 ppm to greater than 2,500 ppm melamine). In addition, other countries have reported detection of melamine in other product categories, such as confections and beverages. We note that there are generally available analytical methods that can reliably detect a level of 1 ppm melamine in some food matrices.Illnesses were reported in different regions of China, including Ganzu, Shaanxi, Ningxia, Jiangsu, Henan, Jiangxi, Hubei, Shandong, Anhui and Hunan. A press release from the Ganzu Health Bureau on September 11, 2008 stated that during the first half of 2008, one hospital in Ganzu treated 16 infants who had kidney stones. The 16 infants varied in age from 5 months to 11 months; some of the cases reportedly developed renal insufficiency. In a record review conducted for the period 2006 to the present, hospitals in Ganzu identified a total of 59 cases of kidney stonesin infants, including 1 infant who died. The cases were located in 24 Ganzu counties and most of the cases were from rural areas. All of the cases occurred in 2008; none were identified in 2006 and 2007. Medical authorities in China indicated that there were 14 cases in which information was available regarding the composition of kidney stones. Twelve of these cases had stones reportedly composed of dihydrate uric acid and urine ammonium. Stones of this composition were reportedly visualized by ultrasound and CT scan, but not by routine x-ray, indicating that some cases may have not been diagnosed.Melamine, in its chainlike "polymerized" form, has been used for decades in manufacturing of dishes, plastic resins, flame-retardant fibers, components of paper and paperboard and industrial coatings. It has only very limited exposure in foods from these food contact substance uses. The estimated level of melamine in food resulting from all of these uses is less than 15 µg/kg (0.015 ppm). Additionally, trichloromelamine is approved for use as a sanitizing agent on food processing equipment and utensils, except for milk containers and equipment. Trichloromelamine readily decomposes to melamine during its use as a sanitizer. Only very low levels of melamine in food would be expected to result from this use. There is no approved melamine use in direct addition to human or animal food in the U.S., nor is it permitted to be used as a fertilizer in the U.S., as it is in some parts of the world.TOXICOLOGICAL STUDY RESULTSThe observed toxic effects of melamine alone in animals in controlled studies occur only following high-dose exposures. All information thus far indicates that melamine appears to be metabolically inactive or inert (i.e., it does not readily undergo any type of metabolic change). This information supports a reasonable probability that all species eliminate the originally ingested substance, melamine or its analogues, and not a metabolite. Some species excrete melamine more slowly than other species. For example, fish excrete melamine more slowly than rodents. In addition, whether adverse effects are observed in some species and not others may vary depending on the level of exposure and which melamine analogues are present. Additionally, one of the bases for differential toxicity to these substances is species-specific rates of elimination.Melamine and its analogues - cyanuric acid, ammelide and ammeline - are assumed to be of equal potency and are referred to collectively below as melamine-analogues. Since there is limited information about the toxicity or pathology of the analogues compared to melamine, it is deemed prudent to make an assumption that these analogues have equaleffects. There is evidence available now that when these melamine analogues, especially cyanuric acid, are available in the kidney simultaneously they can combine to cause renal pathology (see discussion below). The actual extent of renal injury associated with different melamine analogues and the relative concentrations leading to toxicity is under experimental study now.Preliminary work suggests that lattice crystals composed of melamine and cyanuric acid, and possibly other substances, form in the kidney. This has been shown to take place at various dose levels and is a threshold- and concentration-dependent phenomenon that would not be relevant to low levels of exposure to single melamine-type compounds. The combination of melamine and cyanuric acid has been linked to acute renal failure in cats and dogs. Crystals from cats that died from pet food containing melamine and cyanuric acid were comprised of melamine combined with cyanuric acid.1, 2Melamine-cyanurate crystals have been shown to develop in mice, pigs, cats and fish kidneys when dosed with the combination of both melamine and cyanuric acid.2-4The crystals that form in the pigs and fish are identical to those seen in cats. The crystals are a lattice of six molecules -- three of melamine and three of cyanuric acid -- held together by hydrogen bonds.In mammals, the toxicity of melamine alone is low, with a half-life of approximately three to four hours. Available publications report theis the amount that kills most sensitive value for oral 50% lethal dose (LD50one-half of the tested animals) is 3,161 mg/kg bw/d in rats.5, 6 The most recently reported no-observed-adverse-effect-levels (NOAELs) are 63 mg/kg bw/d (13 weeks, oral with feed, in rats); 240 mg/kg bw/d (28 days, oral with feed, in rats); 417 mg/kg bw/d (14 days, oral with feed, in rats); and 1,600 mg/kg bw/d (13 weeks, oral with feed, in mice).6 In addition, the most sensitive calculated NOAELs for oral reproductive and developmental toxicity in rats are 400 mg/kg bw/d (maternal) and 1,060 mg/kg bw/d (fetal), respectively.5, 6 The most commonly observed toxic effects in animal experiments where melamine was administered orally include: reduced food consumption, body weight loss, bladder stones, crystalluria, epithelial hyperplasia of urinary bladder, and lowered survival rate. However, no kidney failure or clinical symptoms of kidney failure were observed from these studies, or in a dog study.7, 8 Additional findings include:Only one oral long-term dog study has been reported. Apart from crystalluria (excretion of crystals in the urine) in thatstudy, no other toxic effects were observed in dogs fed 1,200mg/kg bw/d for one year.5, 7 A short term oral study in catsdosed with melamine alone, cyanuric acid alone or both melamineand cyanuric acid in combination found no clinical pathologyin cats treated with the compounds singly. Crystals formed inthe kidneys of cats exposed to both compounds (64 mg/kg bw/d,total) composed of 32 mg/kg bw/d of each compound.4 No studieswith human subjects have been reported for melamine, but thereare limited data on oral cyanuric acid exposure.9∙Histopathological reports on pets that consumedmelamine-contaminated pet food in 2007 indicate thatintratubular crystal obstruction is the mechanism of renalfailure. There is accumulating experimental evidence thatingestion of food contaminated with melamine and cyanuric acidleads to crystal formation in the kidney and subsequent kidneyfailure. Crystals were found in the kidneys, urinary bladderand urine (crystals are microscopic and thus much smaller thanstones). The mechanism of toxicity has been proposed to besimilar to uric acid nephropathy in humans, where crystalsobstruct renal tubules causing acute renal failure.10, 11∙High (4,500 ppm in the diet which is equivalent to 263 mg/kg bw/d) and continuous (2 years) dietary exposure to melamine incontrolled studies is associated with an increase in theproduction of bladder stones and an increased incidence ofurinary bladder tumors in male rats.6,8 Only rats that hadbladder stones developed tumors in these studies.12∙The NOAEL for stone formation of melamine toxicity is 63 mg/kg bw/d in a 13-week rat study.6 This value is the lowest NOAEL(nontoxic dose) for melamine exposure noted in the publishedliterature and is used with human exposure assessments belowto provide an estimate of human safety/risk.∙In mammals, the toxicity of cyanuric acid (CYA) administered by itself is low. The acute lethal dose (LD) has been reported50as 3,400 mg/kg bw. Kidney toxicity is observed in mice at veryhigh dose (80,000 mg/kg bw/d); rats show kidney toxicity at5,400 mg/kg bw/d). The time taken to clear 50% of anadministered dose: dogs - 2 hours; rats - ½ -1 hour13. SAFETY/RISK ASSESSMENT FOR INFANT FORMULAFDA in collaboration with the Food Safety and Inspection Service (FSIS) of the Department of Agriculture, and in consultation with the Centersfor Disease Control and Prevention (CDC, the Environmental Protection Agency (EPA) and the Department of Homeland Security (DHS) developed a TDI (Tolerable Daily Intake) for melamine and its analogues during the pet food contamination event of 2007.[b] It was based on results from animal studies with melamine and cyanuric acid administered alone. We designated 0.63 mg/kg bw/d as the tolerable daily intake, or TDI. The TDI is defined as the estimated maximum amount of an agent to which individuals in the population may be exposed daily over their lifetimes without appreciable health risk.[c]This TDI was used to provide the basis for the safety/risk assessment in FDA's May 2007 Interim Melamine and Analogues Safety/Risk Assessment for food products from animals fed feed containing melamine compounds. In the present exposure event, the potential risk for toxicity from consumption of infant formula contaminated with melamine and its analogues is far higher than last year's risk of toxicity to humans from consumption of animals that had been inadvertently fed contaminated feed.The previous assumptions that US FDA made in the 2007 risk/safety assessment regarding the pet food contamination episode cannot be applied to the current situation because the contaminated product represents the totality of caloric exposure for most of these infants; the exposure is chronic over months; the persons ingesting the products are infants and toddlers whose renal systems are not yet fully developed; and the exposure is not mitigated by previous passage through the digestive system of an animal. Moreover, several significant gaps in our scientific knowledge about melamine and its analogues toxicity regarding infants exist, including:1.The impact of the presence of more than one melamine analoguewhich has the potential to increase the toxicity of theadulterated infant formula.2.The consequences of continuous use of these infant formulas assole source of nutrition.3.The possibility that these formulations can be fed as the solesource of nutrition to premature infants with immature kidneyfunction and even greater intake of infant formula per unit bodyweight for a longer time period than term infants.Thus, the US FDA cannot establish a level of melamine and its analogues in these products that does not raise public health concerns.[d]SAFETY/RISK ASSESSMENT FOR FOOD AND FOOD INGREDIENTS OTHER THAN INFANT FORMULAThe 2007 estimate of the TDI for melamine and its analogues serves as the starting point for the present risk assessment.[e]That TDI is 0.63 mg/kg bw/d and is based on the results of a 13-week rat study. We describe below the procedure used to calculate a level of melamine and its analogues in food that does not raise public health concerns. A 100-fold safety factor is often accepted as an adequate margin between the lowestno-observed-adverse-effect level (NOAEL) from animal data and the TDI (tolerable daily intake) for humans, such that:63 mg melamine and its analogues/kg-bw/d (NOAEL) divided by 100-fold safety factor=0.63 mg melamine and its analogues/kg-bw/d (TDI).More recent studies2,4 indicate that increased toxicity results from combined exposure to melamine and cyanuric acid. This raises a high degree of uncertainty with regard to the determination of safety/risk.[f] Given these conditions, the FDA has applied an additional 10-fold safety factor, yielding a combined safety factor of 1000-fold, to compensate for these uncertainties.0.63 mg melamine and its analogues/kg-bw/d divided by added 10-fold safety factor = 0.063 mg melamine and its analogues /kg-bw/d.The next step is to convert from a dose of 0.063 mg/kg bw/d to total melamine and its analogues consumed per day.0.063 mg/kg-bw/d x 60 kg/person = 3.78 mg melamine and itsanalogues/person/day.To estimate the level of melamine that does not raise public health concerns,FDA used a worst case exposure scenario in which one-half of a person's total daily dietary intake (typically estimated at 3 kg (composed of liquid [1.5 kg] and solid food [1.5 kg]) is contaminated with melamine and its analogues. The previously determined (see above) total amount of melamine and its analogues/person/day:3.78 mg melamine and its analogues/person/day divided by 1.5 kg of food = the food contamination level that would provide this amount of melamine and its analogues to a 60 kg person per day. Thus, 3.78 mg melamine andits analogues divided by 1.5 kg of food = 2.5 mg melamine and its analogues/kg food.Therefore, if 50% of the diet were contaminated at a level of 2.5 ppm of melamine and its analogues, a person's daily intake would equal 0.063 mg/kg bw/d.dThe current incident has focused on melamine contamination of milk and milk-derived ingredients from China. As illustrated in Table 1, U.S. consumers would be exposed to only 1.1 % of the melamine TDI/10 (0.063 mg/kg bw/d) if all of the major milk-derived ingredients listed below were contaminated at a melamine level of 2.5 mg/kg (2.5 ppm) assuming an average per capita ingredient intake.Table 1U.S. Per Capita Melamine Intakes from Milk-derived Food Ingredients as a % of the TDI/10 if Melamine is Present at 2.5 mg/kgConcentration13Divide % TDI/10 by 100 to determine multiple of TDI/10. TDI is 0.63 mg/kg bw/d.4 Summation of per capita melamine intakes of all eight ingredients.In summary, excluding infant formula and assuming that 50% of the diet is contaminated at a level of 2.5 ppm melamine and its analogs, there is a 1000-fold difference between the estimated dietary exposure (intake) and the level of melamine that does not cause toxicity in animals (NOAEL). Thus, levels of melamine and its analogues below 2.5 ppm in foods other than infant formula do not raise public health concerns.REFERENCES1.Brown CA, Jeong KS, Poppenga RH, Puschner B, Miller DM, EllisAE, Kang KI, Sum S, Cistola AM, Brown SA. Outbreaks of renalfailure associated with melamine and cyanuric acid in dogs andcats in 2004 and 2007. J Vet Diagn Invest 2007; 19:525-531.2.Reimschuessel, R., Gieseker, C., Miller, R.A., Rummel, N., Ward,J., Boehmer, J., Heller, D., Nochetto, C., De Alwis , H.,Bataller, N., Andersen, W., Turnipseed. S. B., Karbiwnyk, C.M. Satzger, R D., Crowe, J., Reinhard, M.K., Roberts,J.F., and Witkowski, M. Evaluation of the renal effects ofexperimental feeding of melamine and cyanuric acid to fish andpigs. Am. J. Vet. Res 69(9):1217-28.. 2008.3.Dobson RL, Motlagh S, Quijano M, Cambron RT, Baker TR, PullenAM, Regg BT, Bigalow-Kern AS, Vennard T, Fix A, ReimschuesselR, Overmann G, Shan Y, Daston GP. Identification andCharacterization of Toxicity of Contaminants in Pet FoodLeading to an Outbreak of Renal Toxicity in Cats andDogs. Toxicol Sci. 2008 Aug 9. [Epub ahead of print].4.Puschner B, Poppenga RH, Lownsteirn LJ, Filigenzi MS, PesaventoPA. Assessment of melamine and cyanuric acid toxicity incats. J Vet Daign Invest 2007, 19:616-624.5.IUCLID Chemical Data Sheet: Substance ID 108-78-1, p. 30-98,February 18, 20006.OECD SIDS Analysis UNEP Publications: Melamine, June 2002.7.Clayton, G. D. and FE Clayton (eds.) Patty's Industrial Hygieneand Toxicology. Vol. 2A, 2B, 2C: Toxicology. 3rd Ed. New York:John Wiley Sons, 1981-1982., p.2771.8.U.S. Department of Health and Human Services: NTP TechnicalReport, TR 245, 1983.9.Allen LM, Briggle TV, Pfaffenberger CD (1982). Absorption andexcretion of cyanuric acid in long-distance swimmers. Drug Metab Rev. 1982;13(3):499-516.10.Conger JD, Falk SA, Guggenheim SJ, Burke TJ. A micropuncturestudy of the early phase of acute urate nephropathy. J Clin Invest 1976; p58:681-9.11.Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A,Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med 2004; 116:546-554.12.Memorandum by CFSAN Cancer Assessment Committee dated March17, 198313.IUCLID Chemical Data Sheet: Substance ID 108-80-5, 65pages, February 18, 2000.。