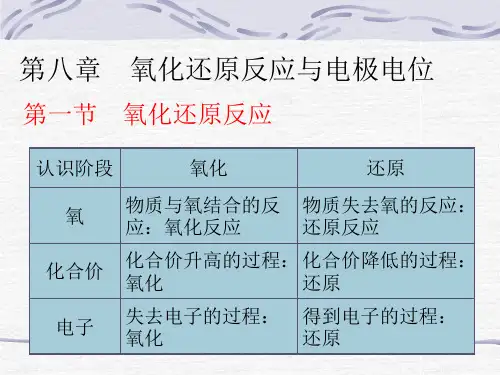
第八章+氧化还原反应3
- 格式:pdf
- 大小:215.25 KB
- 文档页数:14

->电极电位的测量
(一)标准氢电极
比与溶液中H+可达到以下平衡:
2H+ + 2e- H H2
lOOkPa^气饱和了的钳片和氢离子浓度
为lmolL>的酸溶液之间所产生的电势
差就是标准氢电极的电极电位,定为零:
SHE: Pt, H2(l««kPa) JI+(m =1)
(二)标准电极电位标准氢电极与其它各种标准状态下的电极组成原电池,标准氨电极
定在左边,用实验方法测得这
个原电池的电动势数值,就是
该电极的标准电极电位。
比较氧化剂或还原剂的强弱
标准电极电势值越大,电对中氧化型得电 子倾向越大,是强氧化剂;其共觇的还原型失 电子倾向越弱。
标准电极电势值越小,电对中 还原型失电子倾向越大,是强还原剂;其共 的氧化型得电子倾向越小。
判断氧化还原反应自发进行的方向.对角线规则
试判断标准态下反应2Fe2++ i2=2Fe3++2厂自 发进行的方向。
两电对中0°值高的氧化型物质为较强的
6
氧化剂,0值低的还原型物质为较强的还原剂。
Fe 2+ (aq) 0(Fe^/Fe^ ) = 0.77 IV
反应将逆向(由右向左)自发进行。
即:
2Feh + 21- = 2Fe2+ +
1
1 ■
强氧化剂| +强还原剂2
弱还原剂| +弱氧化剂2
bU/I )=0.5355^
【2 (s)+
2e" 广
(aq)
Fe*(aq) + e'。



第八章氧化还原反应和氧化还原滴定法第一节氧化还原反应的基本概念第二节氧化还原反应方程式的配平、教学目的掌握氧化还原反应的基本概念,能配平氧化还原反应方程式二、教材分析(包括重点、难点、关键等)1. 重点:氧化数的概念,方程式的配平2. 难点:氧化数的概念三、教学过程内容安排:第一节课:介绍氧化还原反应的基本概念第二节课:介绍离子- 电子法配平氧化还原反应方程式课堂交流:关于氧化数的理解课堂练习:186页1、3第八章氧化还原反应和氧化还原滴定法、教学目的课堂交流:对电极电势的理解第三节电极电势掌握电极电势的基本概念,能用能斯特方程进行有关计算二、教材分析(包括重点、难点、关键等)1. 重点:理解电极电势的概念,能用能斯特方程进行有关计算2. 难点:电极电势的概念三、教学过程第二节课:介绍能斯特方程内容安排:第一节课:介绍原电池、电极电势第八章氧化还原反应和氧化还原滴定法、教学目的课堂练习:186页4课堂交流:对电极电势的理解第八章 氧化还原反应和氧化还原滴定法、教学目的掌握电极电势在有关方面的应用 二、教材分析(包括重点、难点、关键等)1. 重点:能计算原电池电动势, 判断氧化还原反应的方向, 能确定氧化还原反应 的平衡常数2.难点:判断氧化还原反应的方向,K sp ®, K 稳的计算三、教学过程第一节课:介绍计算原电池电动势,判断氧化还原反应的方向第二节课:介绍确定氧化还原反应的平衡常数介绍K sp ", K 稳的计算课堂练习:186页 12第四节 电极电势的应用课堂交流: 对确定氧化还原反应的平衡常数的理解内容安排:第八章氧化还原反应和氧化还原滴定法第四节元素电势图及其应用、教学目的掌握元素电势图及其应用二、教材分析(包括重点、难点、关键等)1. 重点:元素电势图及其应用2. 难点:元素电势图及其应用三、教学过程内容安排:第一节课:介绍元素电势图第二节课:介绍元素电势图的应用课堂交流:对元素电势图的理解第八章氧化还原反应和氧化还原滴定法、教学目的第二节课:氧化还原滴定曲线课堂交流:氧化还原滴定曲线第六节氧化还原滴定法了解氧化还原滴定法中指示剂类型及氧化还原滴定法特点二、教材分析(包括重点、难点、关键等)1. 重点:氧化还原滴定曲线及氧化还原滴定法指示剂2. 难点:氧化还原滴定曲线三、教学过程内容安排:第一节课:氧化还原滴定法特点第八章氧化还原反应和氧化还原滴定法第六节氧化还原滴定法、教学目的了解氧化还原滴定法中指示剂类型二、教材分析(包括重点、难点、关键等)1. 重点:氧化还原滴定法指示剂2. 难点:氧化还原滴定法指示剂三、教学过程内容安排:第一节课:氧化还原滴定法指示剂第二节课:常用的氧化还原滴定法课堂交流:氧化还原滴定法指示剂第八章氧化还原反应和氧化还原滴定法第七节常用的氧化还原滴定法、教学目的熟练掌握氧化还原滴定法主要方法和应用二、教材分析(包括重点、难点、关键等)1. 重点:氧化还原的预处理2. 难点:氧化还原的预处理三、教学过程内容安排:第一节课:重铬酸钾法标准溶液配制,预处理第二节课:重铬酸钾法应用示例课堂交流:重铬酸钾法课堂练习:186 页17第八章 氧化还原反应和氧化还原滴定法、教学目的第二节课:碘量法应用示例课堂交流:第七节 常用的氧化还原滴定法熟练掌握氧化还原滴定法主要方法和应用 二、教材分析(包括重点、难点、关键等)1. 重点:碘量法标准溶液配制,应用示例2. 难点:碘量法应用示例 三、教学过程课堂练习: 225 页 18内容安排: 第一节课:碘量法标准溶液配制第九章配位平衡和配位滴定法第一节配合物的组成和命名、教学目的1、掌握配位化合物的定义、组成、命名和分类、教材分析(包括重点、难点、关键等)1. 重点:配位化合物的定义、组成、命名和分类2. 难点:中心原子、配位原子、配位数三、教学过程内容安排:第一节课:介绍配位化合物的定义、组成第二节课:介绍配位化合物的命名和分类课堂交流:对中心原子、配位原子、配位数的理解课堂练习:218 页1 、2第九章配位平衡和配位滴定法第二节配位化合物的价键理论、教学目的1、掌握配位化合物价键理论、教材分析(包括重点、难点、关键等)1. 重点:配位化合物价键理论2. 难点:配位化合物价键理论三、教学过程内容安排:第一节课:介绍配位化合物的命名和分类第二节课:介绍配位化合物价键理论课堂交流:对配位化合物价键理论的理解课堂练习:349 页5第三节配位平衡第四节EDTA 及其与金属离子的配合物、教学目的1、掌握配位平衡和配位平衡常数的意义及其有关计算2、理解配位平衡的移动及第九章配位平衡和配位滴定法与其他平衡的关系。



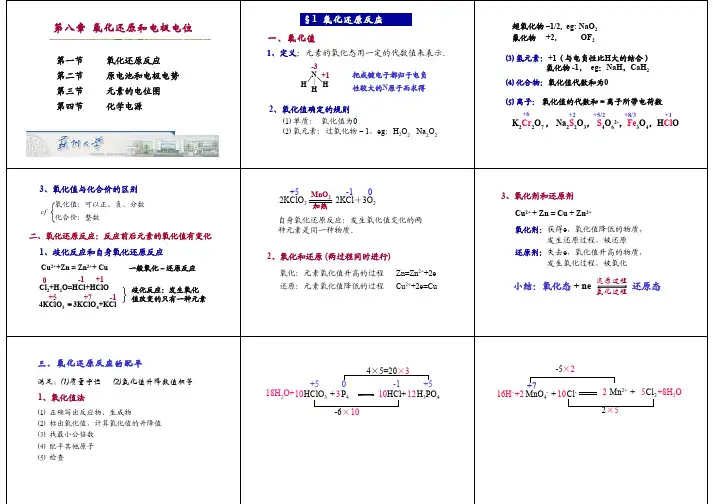
第⼋章-氧化还原反应与氧化还原滴定习题及答案第⼋章氧化还原反应与氧化还原滴定习题1.是⾮判断题1-1氧化数在数值上就是元素的化合价。
1-2 Na 2S ,Na 2S 2O 3,Na 2SO 4和NaS 4O 6中,硫离⼦的氧化数分别为-2,2,4,6和+5/2 。
1-3 NH 4+中,氮原⼦的氧化数为-3,其共价数为4。
1-4氧化数发⽣改变的物质不是还原剂就是氧化剂。
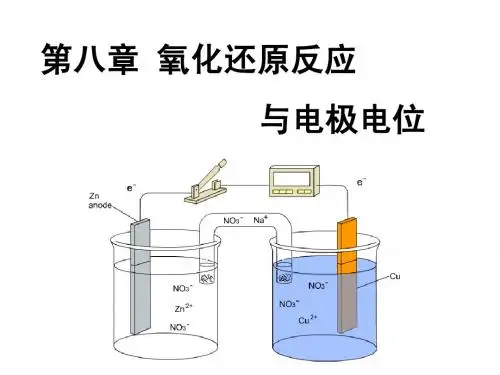
1-5任何⼀个氧化还原反应都可以组成⼀个原电池。
1-6两根银丝分别插⼊盛有 mol ·L -1和 1 mol ·L -1 AgNO 3溶液的烧杯中,且⽤盐桥将两只烧杯中的溶液连接起来,便可组成⼀个原电池。
1-7在设计原电池时,θ?值⼤的电对应是正极,⽽θ?值⼩的电对应为负极。
!1-8原电池中盐桥的作⽤是盐桥中的电解质中和两个半电池中过剩的电荷。
1-9半反应NO 3- + H + + e ?NO + H 2O 配平后,氧化态中各物质的系数依次为1,4,3。
1-10在碱性介质中进⾏的反应CrO 2-+Cl 2+OH -?CrO 42-+Cl -+H 2O 被配平后反应⽣成物CrO 42-的系数分别为8和2。
1-11对电极反应S 2O 82-+2e2SO 42- 来说,S 2O 82- 是氧化剂被还原,SO 42-是还原剂被氧化。
1-12原电池中,电⼦由负极经导线流到正极,再由正极经溶液到负极,从⽽构成了回路。
1-13⾦属铁可以置换CuSO 4溶液中的Cu 2+,因⽽FeCl 3溶液不能与⾦属铜反应。
1-14标准电极电势表中的θ值是以氢电极作参⽐电极⽽测得的电势值。
1-15电极电势表中所列的电极电势值就是相应电极双电层的电势差。
1-16某电对的标准电极电势是该电对与标准氢电极组成原电池时的原电池电动势。
1-17电极反应为Cl2+2e2Cl -的电对Cl 2/Cl -的E θ=;电极反应为12Cl 2+e Cl -时θ(Cl 2/Cl -) …=1/2×=。