Ductal Carcinoma In Situ
- 格式:pdf
- 大小:482.92 KB
- 文档页数:3

磁共振成像对乳管内病变的诊断价值胡华宇1,李席如2*1.南开大学医学院,天津300071;2.解放军总医院第一医学中心普外科,北京100853;*通讯作者李席如 ***************【关键词】乳腺肿瘤;磁共振成像;综述【中图分类号】R730.42;R737.9 【DOI】10.3969/j.issn.1005-5185.2020.07.019动态增强磁共振(dynamic contrast enhanced-magnetic resonance imaging,DCE-MRI)的软组织分辨能力较高,且无创、无辐射,能提供病灶血供分析,近年来越来越多地应用于乳腺疾病检查,结合扩散加权成像(DWI)、磁共振波谱成像(MRS)可以从形态及功能等方面分析病灶,对乳腺癌的诊断及预后有重要价值[1]。
导管原位癌(ductal carcinoma in situ,DCIS)是一种未突破基底膜的乳管内病变,占乳腺癌的2%~30%[2],且有发展为浸润癌的倾向,早期治疗会使DCIS患者受益。
然而,导管内病变病灶隐匿,有时不能被传统的乳腺检查检出或鉴别[3]。
本综述拟探讨MRI对乳管内病变的诊断价值。
1 MRI对乳管内病变的诊断乳管内病变主要包括DCIS、导管内乳头状瘤、乳腺导管扩张症(又称为浆细胞性乳腺炎)、乳管急慢性炎症等。
导管内病变以乳头溢液为主要临床表现。
以往将乳腺导管造影作为病理性乳头溢液患者的首选检查,但它不易在阳性导管图上区分病变的良恶性,Meta分析显示其合并敏感度和特异度分别为69%和39%[4];该检查为侵入性,耗时较长,要求检查当天必须有乳头溢液,限制了其在临床上的应用。
既往钼靶对乳头溢液的诊断敏感度为9.5%~26.0%,特异度为38.0%~98.4%[5-6],故钼靶检查阴性不能排除任何诊断。
超声诊断有病理性乳头溢液的导管内病变比钼靶检查更准确,敏感度为46.4%~80.0%,特异度为42.9%~87.0%[5-8],它能更清楚地观察扩张的导管及管内低回声内容物,然而部分DCIS在超声中常为阴性而漏诊。

cyclin D1与人类肿瘤现代学者在观察肿瘤的不协调生长和增生时,发现了与细胞周期调控有关的蛋白,并认为肿瘤与细胞周期调控失常有着密切联系。
cyclin D1作为细胞周期调节因子之一,其过度表达是多种人类原发性肿瘤的特征,对肿瘤的诊断和预后判断具有重要意义。
一、正常细胞周期调控细胞周期分为G1、S、G2和M 4阶段,由3类因子进行精密调控,它们分别是周期素依赖性激酶(cyclin-dependent kinases,CDKs)、周期素(cyclins)和周期素依赖性激酶抑制因子(cyclin-dependent kinases inhibitors,CKIs),其中CDKs处于调控中心地位,cyclins起正调节作用,CKIs发挥负调节作用。
1.周期素:目前已在哺乳动物细胞中分离出8类主要的周期素,连同亚类共11种,分别是A、B1、B2、C、D1、D2、D3、E、F、G 和H。
周期素含量随细胞周期而变化,不同的周期素在其相应周期时相达到含量和活性的高峰,激活相应的CDKs,随后迅速降解失活[1]。
例如:cyclin C、D1-3和E在G1期达到最大活性,并调节G1期向S 期的过渡。
2. 周期素依赖性激酶及其抑制因子:CDKs(CDK1-7)是一组依赖于周期素,在细胞周期调节中起关键作用的蛋白激酶,其活性也随周期而变化。
如CDK4、6主要在G1期与D型cyclins相结合。
与细胞周期调控有关的还有CKIs,分为两类:一类是Kip/Cip家族,包括3种结构相关的蛋白(p21、p27和p57);另一类是INK4蛋白家族,由4种相似蛋白(p15、p16、p18和p19)构成。
Kip/Cip家族能结合并抑制大多数cyclin-CDK复合物,而INK4分子则可能是含D型cyclins 复合物的特异性抑制因子。
二、肿瘤细胞周期调控在肿瘤细胞中,推动细胞周期的蛋白如周期素常过度表达,而减缓细胞分裂的蛋白如CKIs却常常失活。

乳腺基底样癌乳腺癌是一组异质性肿瘤,具有相同病理诊断及预后特征的乳腺癌患者常表现出明显不同的临床结果,这可能是目前所采用的乳腺癌分类方法造成的,因为目前的分类主要建立在形态学表现上。
2000年,Perou等采用cDNA微阵列方法研究发现,可以按基因表型对乳腺癌进行重新分类,将乳腺癌分为四种类型:ER+/腔样型(ER+/luminal)、正常乳腺型(nomal breast-like)、基底样型(basal-like subtype)和Her2/neu+型,其中后两者预后较差。
(Perou CM, Serlie T, Eisen MB. et al. molecular portraits of human breast tumours. Nature. 2000;406:747-752),2001年,Sorlie等又将腺腔型分为A、B两型(B型还可分为2型)(Sorlie T, C, Tibshirani R. gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS. 2001;98(19):.)。
后继研究表明,这一分类方法与乳腺癌患者的预后及治疗有较好的相关性。
一、基底细胞样癌的定义:最初,基底样癌的定义是建立在基因表型上的,表现为在基因水平高表达CK5、CK6、金属硫蛋白1X, 脂肪酸结合蛋白7、SFRP1, c-kit,低表达fibronectin 1和mucin1。
Sotiriou等又将其分为两型,A型基底样癌:高表达基质金属蛋白酶7和与细胞增殖相关的基因如topoidomerase Ⅱα, MAD2L1, CDC2 和PCNA,说明具有较高的增殖活性;基底样B型:高表达AP-1相关基因如c-fos, c-jun, fos B, activating transcription factor 3, caveolin 1 和caveolin 2, hepatocyte growth factor and transforming growth factorβreceptor Ⅱ(Sotiriou C, Neo SY, L. et al. breast cancer classification and prognosis based on gene expression profiles from a population-based study. PNAS. 2003;100(18):.)。


原位癌与浸润癌病理划分标准概述及解释说明1. 引言1.1 概述引言部分将对本文的主题进行概述。
在该文章中,我们将重点讨论原位癌与浸润癌的病理划分标准。
病理划分是医学领域中重要的分类方法之一,它有助于医生对不同类型的癌症进行诊断和治疗。
1.2 文章结构本文采用以下结构进行组织:引言部分将介绍原位癌与浸润癌的概念,并说明文章的目的。
接下来,我们将详细探讨原位癌和浸润癌的定义、特点以及分类。
随后,我们会比较并阐述两者之间的区别与联系。
最后,通过总结得出结论。
1.3 目的本文的目的是为读者提供关于原位癌和浸润癌病理划分标准方面的基本知识。
我们希望通过全面而明确地解释这两种类型肿瘤的定义、特点和分类方式,增进读者对原位癌与浸润癌之间关系的理解,并促使其对相关领域展开更深入的学习和研究。
在了解了文中“引言”部分内容后,请问还有什么可以帮助您的吗?2. 原位癌的定义与特点2.1 定义原位癌是一种生长在体内组织中并没有侵犯邻近组织或扩散至远处器官的肿瘤。
字面意思上,原位(in situ)指的是肿瘤细胞仍停留在其初始发生的位置,并未穿过基底膜进入周围组织。
2.2 特点原位癌具有以下几个特点:1. 组织内部局限性:原位癌通常只限于原发发生的组织内部,尚未通过侵袭扩散到其他部位或器官。
2. 病理形态上可见异常增殖及细胞变异:通过显微镜观察,可以看到肿瘤细胞在形态和结构上出现明显的异常改变。
3. 未穿透基底膜:肿瘤细胞仍停留在起源位置,没有突破基底膜,并且未进入周围正常组织。
4. 尚无血管侵犯和淋巴结转移:由于原位癌尚未突破基底膜,所以很少涉及血管系统和淋巴系统的侵犯,也没有远处器官的转移。
2.3 分类原位癌可按照不同组织类型和部位进行分类,下面是一些常见的原位癌分类:1. 乳腺原位癌:包括导管内癌和导管外癌,其中最常见的是导管内癌(ductal carcinoma in situ, DCIS)。
2. 子宫颈原位癌:也被称为宫颈上皮内瘤变(cervical intraepithelial neoplasia, CIN),根据病理程度可分为CINⅠ、CINⅡ和CINⅢ。


非肿块型乳腺病变的超声诊断李晔;王知力【摘要】Many breast lesions show non-mass-like lesions instead of presenting with nodules or lumps. High-resolution ultrasonography can identify the non-mass-like breast lesions which may be ignored in clinic. This review elucidates various ultrasonographic findings of the non-mass-like breast lesions, and the correlation of ultrasonographic findings and sonographic-pathologic by elastography, Doppler techniques and other radiological technologies.%很多乳腺病变并不表现为结节或肿块,而是表现为非肿块型病变.高分辨率超声能够识别临床上被掩盖的非肿块型乳腺病变.本文就非肿块型乳腺病变的不同超声表现以及通过采用弹性成像技术、多普勒技术和其他影像学手段反映的其超声表现与病理类型的相关性作一简要综述.【期刊名称】《解放军医学院学报》【年(卷),期】2015(036)009【总页数】3页(P957-959)【关键词】非肿块型乳腺病变;超声检查;诊断【作者】李晔;王知力【作者单位】解放军总医院超声诊断科,北京 100853;解放军总医院超声诊断科,北京 100853【正文语种】中文【中图分类】R730.4随着高频超声技术的进步,越来越多的非肿块型乳腺病变可以被发现,但其超声图像特征还没有得到充分的认识,诊断及鉴别诊断正确率还很低。
恶性非肿块型乳腺病变更是诊断的难点和重点。


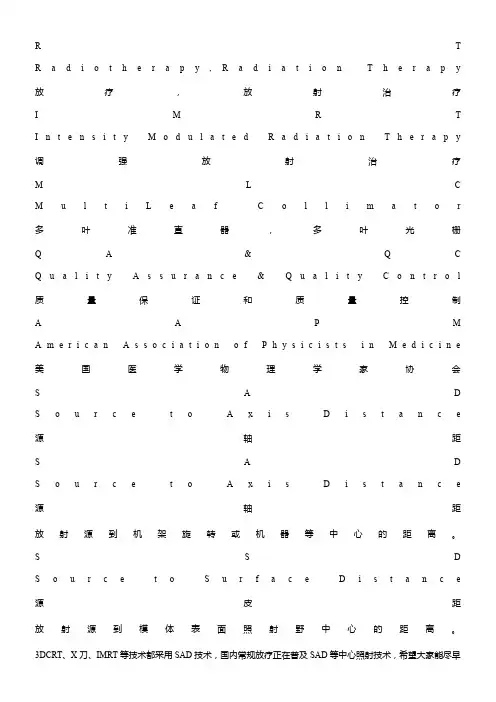
R T R a d i o t h e r a p y,R a d i a t i o n T h e r a p y 放疗,放射治疗I M R T I n t e n s i t y M o d u l a t e d R a d i a t i o n T h e r a p y 调强放射治疗M L C M u l t i L e a f C o l l i m a t o r 多叶准直器,多叶光栅Q A&Q C Q u a l i t y A s s u r a n c e&Q u a l i t y C o n t r o l 质量保证和质量控制A A P M A m e r i c a n A s s o c i a t i o n o f P h y s i c i s t s i n M e d i c i n e 美国医学物理学家协会S A D S o u r c e t o A x i s D i s t a n c e 源轴距S A D S o u r c e t o A x i s D i s t a n c e 源轴距放射源到机架旋转或机器等中心的距离。
S S D S o u r c e t o S u r f a c e D i s t a n c e 源皮距放射源到模体表面照射野中心的距离。
3DCRT、X刀、IMRT等技术都采用SAD技术,国内常规放疗正在普及SAD等中心照射技术,希望大家能尽早放弃S S D技术,只在某些特定情况下采用S S D技术。
P D D P e r c e n t a g e D e p t h D o s e 百分深度剂量定义为射野中心轴上某一深度处的吸收剂量率与参考点深度处剂量率的百分比。
对于高能X(γ)射线,参考深度一般取在射野中心轴上最大剂量点深度处。
T M R T i s s u e M a x i m u m R a t i o 组织最大剂量比定义为模体中射野中心轴上任意一点的剂量率与空间同一点模体中射野中心轴上最大剂量点深度处同一射野的剂量率之比。

乳腺大汗腺癌临床和病理学特征研究目的:探讨乳腺大汗腺癌(AC)的临床和病理学特征。
方法:筛选出的8例乳腺大汗腺癌进行临床病理分析,采用过碘酸雪夫(PAS)、阿辛蓝(AB)染色及免疫组织化学A-P法检测GCDFP-15、Mucin-1、Ki-67、雄激素受体(Androgen receptor,AR)、雌激素受体(estrogen receptor,ER)、C-erbB-2。
结果:8例均发生于中老年女性,因发现乳房包块就诊。
组织学8例均为高级核,其中大汗腺导管原位癌(ADCIS)1例,ADCIS伴浸润性大汗腺癌(IAC)5例,IAC伴非大汗腺型DCIS灶2例。
镜下主要有2种形态:A型,胞质嗜伊红染的粗颗粒状;B型,胞质淡染,呈细粉尘颗粒状,如毛玻璃样。
8例AC的PAS染色阳性,AB 染色在ADCIS阴性,在IAC可有50%细胞阳性,并同时表达GCDPF-15,AR,Mucin-1及Ki-67;除1例外,ER均不表达。
结论:乳腺大汗腺癌的临床表现和大体形态与非乳腺大汗腺癌相似,多发生于单侧并且多数伴有同侧腋窝淋巴结转移,预后较好。
其诊断主要依靠病理:(1)特征性的AC型细胞;(2)GCDPF-15阳性率高;(3)AR阳性率高,ER阳性率低。
大汗腺乳腺癌是浸润性导管癌的一种罕见的亚型,国内外文献报道约占乳腺癌的0.3%~1%[1]。
本院近年诊治8例乳腺大汗腺癌(apocrine carcinoma,AC),均经病理诊断证实,现结合文献进行临床、病理及免疫组织化学观察以探讨其特征及预后。
1 资料与方法1.1 一般资料复习1997-2003年安徽医科大学第一附属医院病理科1562例乳腺癌档案切片,筛选出HE形态拟为AC的8例标本进行形态学和免疫组化检查,均为女性,平均年龄(49.0±13.1)岁;部位:左乳3例,右乳5例;肿块直径1.5~5 cm。
1.2 病理检查所有组织均为4%甲醛固定和石蜡包埋,常规制片,其中3 μm 不涂胶切片行HE,过碘酸雪夫(PAS)和阿辛蓝(AB)染色,免疫组织化学标记采用S-P法,所用一抗为鼠抗人GCDFP-15、Mucin-1、Ki-67、AR、ER、c-erbB-2单克隆抗体,均购自北京中山生物技术有限公司,以PBS代替一抗做阴性对照。
86·中国CT和MRI杂志 2024年5月 第22卷 第5期 总第175期【通讯作者】钱丽霞,女,主任医师,主要研究方向:神经影像、胸腹及骨关节影像。
E-mail:**************Prognostic Value of Preoperative PunctureCHINESE JOURNAL OF CT AND MRI, MAY. 2024, Vol.22, No.5 Total No.175线圈,采用俯卧位,头先进,双侧乳房自然悬垂于线圈内。
扫描范围包含双侧乳腺组织及腋窝,扫描序列为:平扫横断面和矢状面扫描:使用TIRM序列,TR 3570 ms,TE 70 ms,层厚3mm,层间距1.5,FOV 350mm×350mm,采集矩阵350×450。
横轴面Resolve-DWI扫描,TR 5700 ms ,TE 66 ms,层厚4.5mm,采集矩阵86×190,分段采集次数3,b值分别取0s/mm2和800s/mm2。
MRI动态增强多期扫描:采用超快速并行采集技术(CAIPIRINHA)、水脂分离压脂技术(Dixon)及时间分辨交叉随机轨迹成像(TWIST)三种技术的容积插入法屏气扫描检查(CDT-VIBE)序列,TR 5.65 ms,TE 3.72 ms,FOV 360mm×360mm,矩阵240×180,层厚2.0mm,采用无间隔扫描,翻转角10°,共采集图像26期,单期时间分辨率约为13 s,总采集时间6分钟左右。
横轴面薄层延迟期扫描:采用横轴面VIBE序列,TR 4.3 ms,TE 1.7 ms,FOV 320mm×320mm,矩阵350×450,层厚0.8 mm,翻转角10°。
动态增强扫描开始前按0.1mmoL/Kg体重注射钆布醇注射液,流率2.5mL/s,后追加15mL生理盐水以同样流率注射。
全民健康助力全面小康浅谈乳腺原位癌曹威北京大学肿瘤医院暨北京市肿瘤防治研究所乳腺癌预防治疗中心,恶性肿瘤发病机制及转化研究教育部重点实验室,北京100142乳腺原位癌曾被认为是乳腺上皮内的肿瘤性病变向浸润性乳腺癌过渡的一种病理类型,临床上较为罕见。
但近年来,随着人民健康意识的不断提高和政府在卫生领域投入的持续加大,乳腺癌筛查不仅在大城市基本普及,中小城市和农村地区也在逐步开展,随之而来的是乳腺原位癌的检出率开始大幅度提升,逐渐成为乳腺癌的常见类型之一。
本文从发病情况、临床特征、治疗与预后等方面对乳腺原位癌作一简要阐述,以期对乳腺原位癌有一个初步认识。
1乳腺乳腺原位癌的基本概念原位癌的基本概念乳腺的主要结构包括由腺泡组成的、用于产生乳汁的小叶系统,由开口于乳头的乳导管及其各级分支组成的导管系统,以及填充在小叶和导管之间的脂肪、纤维、血管、淋巴管等间质组织。
其中乳导管和小叶主要由上皮细胞组成,在上皮细胞外有一层基底膜。
如果乳腺的癌变局限于上皮细胞而没有突破基底膜,没有侵犯乳腺小叶之间的间质,就称为乳腺原位癌。
根据组织来源的不同,乳腺原位癌又分为导管原位癌和小叶原位癌。
与之相对的是肿瘤细胞一旦突破基底膜接触到间质的淋巴管和血管,乳腺癌就存在淋巴和远处转移的风险,即浸润性乳腺癌。
2乳腺导管原位癌导管原位癌是乳腺原位癌的主要病理类型,2012年乳腺肿瘤世界卫生组织(WHO )组织学分类中将其定义为“一种局限于乳腺导管-小叶内的肿瘤性病变,其特征是上皮细胞增生,细胞非典型性从轻微到明显,有发展为浸润性乳腺癌的倾向,但不是一定会进展为浸润性乳腺癌”。
由此可见,导管原位癌被认为是浸润性乳腺癌的前期病变,如不加以治疗,相当一部分患者会进展为浸润性导管癌,从而威胁患者健康与生命。
有研究曾追踪了28例因误诊为良性肿瘤而仅行病灶切除活检的导管原位癌,中位随访28年后发现共有11例(39%)患者发展成为浸润性癌,且所有肿瘤都与最初活检发现的导管原位癌在同一象限,其中5例患者死于转移性乳腺癌[1]。
乳腺硬化性腺病1例并献复习浙江大学医学院附属第一医院,浙江杭州 310003关键词:乳腺增生性病变硬化性腺病病例:患者女性,37岁,1年前无明显诱因下自检发现右乳肿块,无肿块区疼痛、发热,无局部皮肤突起、凹陷、红肿、破溃,未予以重视。
半年前行粗针穿刺,术后病理示:乳腺增生症。
此后定期随访复查,现患者自觉右乳肿块区偶有疼痛,不剧可忍。
10余年前阑尾切除术后,余既往史、个人史、家族史无殊。
查体:右乳4点可及约3cm肿块,质中,界欠清,活动欠佳,皮肤未见明显水肿、发红、凹陷;余对侧乳房未及明显异常,双侧腋窝未及肿大淋巴结。
行钼靶检查示“双乳致密型乳腺”,行超声检查示“右乳混合回声灶,BI-RADS 4a类”。
行乳腺MRI增强扫描示:T1WI呈低信号,T2WI压脂相呈稍高信号,内见多发小类圆形高信号影,DWI呈不均匀稍高信号,范围约1.1*0.9cm,增强扫描可见不规则团片状明显强化,时间信号曲线呈平台型;病灶形态不规则,见多发毛刺影。
结论考虑“右乳内下象限异常信号灶,拟BI-RADS 4b类”。
排除禁忌后行手术切除,术后病理示“(右乳肿块)乳腺增生症,小灶呈硬化性腺病改变;导管囊性扩张伴大汗腺化生,区域上皮生长活跃;P120(+),Ki-67(低),ER(+),PR (+),E-cadherin(+),Calponin(CP)(+),S-100(+)”。
讨论:硬化性腺病(Sclerosing adenosis, SA)属于乳腺腺病的一种,2012版世界卫生组织(WHO)乳腺肿瘤分类中将硬化性腺病、大汗腺腺病、微腺腺病及放射状瘢痕 / 复杂性硬化性病变归于乳腺良性上皮性病变[1]。
SA好发于中青年女性,中位年龄为47岁[2]。
临床上大部分患者为体检发现,部分患者可出现乳腺疼痛、乳房肿块,病变可表现为双侧[3],可不对称,边界欠清,质硬,活动度可,病灶在病程中可增大、缩小甚至消失。
SA起源于终末导管-小叶单位,以小叶为中心,腺泡、肌上皮及结缔组织等间质增生硬化,排列紊乱,导致小叶不同程度的受压变形、改建,形成假浸润的表现和神经周围侵犯,一般界限清楚,是最常见的容易误诊为浸润性癌的良性病变[4]。
ADC值对乳腺导管原位癌侵袭成分的研究价值郭宏兵;刘菲;史新乐;赵一林;邹殿俊【摘要】目的本研究目的是应用最小ADC值和不同ADC值对导管原位癌(Ductal Carcinoma in Situ,DCIS)侵袭成分进行术前预测评估.方法选取2014年6月至2016年10月经病理证实的69例女性DCIS患者70个病灶进行本次试验.所有患者均行MRI检查,从多个感兴趣区测量值中选出最小和最大ADC值.同时计算最大ADC值和最小ADC值间的差值,即ADC差值,后比较DCIS和DCIS侵袭成分的最小ADC值和差值ADC.结果 DCIS侵袭性成分最小ADC值明显低于DCIS,P=0.0037.DCIS侵袭性成分差值ADC值明显高于DCIS,P=0.0007.最小ADC值与差值ADC值具有明显负相关性(r=0.65).ROC分析显示最小ADC值最有效阈值低于1.1×10-3mm2/s,有助于鉴别DCIS侵袭性成分和DCIS,敏感性和特异性分别为72%和77%.ADC差值最有效阈值高于0.23×10-3mm2/s,同样,有助于鉴别DCIS侵袭性成分和DCIS,敏感性和特异性分别为68%和76%.结论最小ADC值和差值ADC有助于发现侵袭成分,确定侵袭成分有助于术前决定是否清扫前哨淋巴结和腋窝淋巴结.%Objective The purpose of this study is to discuss the diagnostic value of different ADC values on ductal carcinoma in situ (DCIS) so as to evaluate the preoperative prediction invasive component. Methods A total of 69 DCIS female patients with 70 lesions confirmed by pathology during June 2014 and October 2016 were included in this study; all of them underwent preoperative MRI. We selected the highest and lowest ADC value from the region of interest, and calculated the difference value between them. The differences between the DCIS and DCIS invasive component were compared. Results The minimum ADC value in DCISinvasive component was much lower than that of DCIS (P=0.0037). The difference value between maximum ADC and minimum ADC in DCIS invasive component was much higher than that of DCIS (P=0.0007). There was negative correlation between minimum ADC value and difference value (r=0.65). ROC showed that the effective threshold-value of ADC maximum value less than 1.1×10-3mm2/s was contributed to identification of DCIS invasive component and DCIS, with sensibility and specificity of 72% and 77%. Similarly, the effective threshold-value of difference value of ADC higher than 0.23×10-3mm2/s was also contributed to identification of DCIS invasive component and DCIS, with sensibility and specificity of 68% and 76%. Conclusion Both the minimum ADC value and the difference value between the minimum and maximum are helpful for identifying of DCIS invasive component and DCIS, which can help to determine if the sentinel lymph node and axillary lymph node could be swept.【期刊名称】《中国医疗设备》【年(卷),期】2018(033)005【总页数】4页(P76-79)【关键词】乳腺导管原位癌;乳腺X线摄影;彩色多普勒超声;磁共振成像;病理组织学【作者】郭宏兵;刘菲;史新乐;赵一林;邹殿俊【作者单位】河北北方学院附属第一医院影像科,河北张家口 075000;河北北方学院附属第一医院影像科,河北张家口 075000;河北北方学院附属第一医院影像科,河北张家口 075000;河北北方学院附属第一医院影像科,河北张家口 075000;河北北方学院附属第一医院影像科,河北张家口 075000【正文语种】中文【中图分类】R730.44引言乳腺导管原位癌(Ductal Carcinoma in Situ,DCIS)是导管内恶性上皮细胞增殖,尚未侵袭到基底膜。
乳腺多形性小叶癌7例临床病理及免疫组化分析张伟;刘霞;李佳嘉;张帆【摘要】Purpose To investigate the clinical and morphological features as well as immunophenotype of pleomorphic lobular carcino-ma ( PLC) . Methods Seven cases of PLC were retrieved from 34 cases of invasive lobular carcinoma. The clinical data, histologic features, immunohistochemical findings were analyzed. Results The mean age of the patients was 56. 6 years, with a range of 47 to 74 years. PLC might be nodular mass in general macroscopy. Histologically, PLC retained classical or non-classical single cells, but exhibited a greater degree of cellular atypia and pleomorphism and a higher mitotic rate than classic invasive lobular carcinoma ( ILC) . PLC was accompanied by ductal carcinoma in situ ( DCIS) and pleomorphic lobular carcinoma in situ ( PLCIS) . Immunohistochemistry and HER-2 FISH were detected. PLC showed E-cadherin negative (7/7) and p120 cytoplasmic positive (7/7), ER (5/7), PR (5/7), and HER-2 (7/7) mostly negative. 5 cases were characterized by triple negative breast cancer, two of which are basal-like pheno-type. Some cases could be observed nerve invasion (3/7) and axillary lymph node metastasis (4/7). Conclusion PLC is a very rare subtype of invasive lobular carcinoma. In some cases there are nerve invasion and lymph node metastasis. The diagnosis of PLC de-pends on the pathological morphological and immunohistochemical markers.%目的:探讨乳腺多形性小叶癌( pleomorphic lobular carcinoma, PLC)的临床病理及免疫表型特点。
从原位癌到微小浸润:乳腺肿瘤的临床病理分析及对外科治疗的影响王一澎;王靖;王翔;陈国际;郭文青;张丽华;马沛卿;张扬;王仲照;方仪;宣立学;高纪东【摘要】背景对乳腺原位癌(CIS)是否应进行前哨淋巴结活检(SLNB)目前仍处于争议中。
临床诊疗中常遇到:一些在术前穿刺或术中活检被诊断为原位癌且未进行SLNB的患者,术后却经病理确诊为乳腺微小浸润性癌(MIBC)。
此时,是否二次手术进行腋窝淋巴结状态评估对外科医师来说是一个困难的选择。
一方面,MIBC淋巴结转移风险尚不明确;另一方面,再次手术时SLNB的准确性及可操作性往往受到质疑,多数情况下腋窝淋巴结清扫成为唯一的选择。
目的识别原位癌伴发微小浸润的危险因素;比较CIS和MIBC腋窝淋巴结的转移风险;探索选择合适的病例直接进行术中前哨淋巴结活检以避免二次手术的合理性。
方法对接受手术且经病理确诊的乳腺原位癌(493例)及微小浸润癌(199例)患者的临床病历资料进行回顾性的统计分析;采用Pearson卡方检验和Fisher确切概率法进行组间比对;通过单因素分析和多因素Logistic回归识别原位癌伴发微小浸润的危险因素。
结果原位癌组中出现4例小叶原位癌(LCIS),其余均为导管原位癌(DCIS),而MIBC所伴发的原位癌均以DCIS为主。
单因素分析显示,X线片BI-RADS≥4级的钙化,肿瘤﹥2.5 cm,高级别DCIS,ER(-),PR (-),HER-2(+++)是原位癌伴微小浸润的危险因素(P均﹤0.05);Ki-67≥20%也可能与发生微小浸润有关(P=0.057)。
使用Logistic回归将重要协变量(年龄)与上述危险因素一起进行多因素分析显示,年龄﹥50岁(P=0.034),肿瘤﹥2.5 cm(P=0.033),高级别DCIS(P=0.011)等是原位癌伴微小浸润的独立危险因素。
此外,相对于2.0%的原位癌淋巴结转移概率,MIBC的淋巴结转移概率为5.5%,二者比较差异具有统计学意义(P=0.029)。
T h e ne w engl a nd jour na l o f medicinen engl j med 374; january 282016390Ductal Carcinoma In SituThis interactive feature addresses the approach to a clinical case. A case vignette is followed by specific options, neither of which can be considered correct or incorrect. In short essays, experts in the field then argue for each of the options. Readers can participate in forming community opinion by choosing one of the options and, if they like, providing their reasons.case vignetteA Woman with Ductal Carcinoma In SituAndrea L. Merrill, M.D.Debbie is an otherwise generally healthy 54-year-old postmenopausal woman who presents to your breast surgery clinic one afternoon with a new diagnosis of ductal carcinoma in situ (DCIS) in her right breast. Her medical history is notable only for mild hypertension and hypothyroidism. Her current medications include hydrochloro-thiazide, levothyroxine, and a multivitamin. She is an eighth-grade math teacher and is married, with two children. She has never smoked, and she drinks one or two glasses of wine per week. Her family history is notable for breast cancer in her mother at the age of 68 years.She has undergone yearly screening mam-mography since she was 40 years of age. Last month, she had her annual screening and a small cluster of microcalcifications was found. A biopsy of the area revealed low-grade, estrogen- and progesterone-receptor–positive DCIS.This new diagnosis of DCIS has caused marked anxiety for Debbie. She has spent time researching her condition on the Internet as well as speaking with friends and family. She tells you that she feels overwhelmed by the various treatment options. She states a strong preference to avoid mastectomy, if possible, and asks wheth-er she needs radiation therapy. She also states that she read a recent article that suggested that she may not need any treatment. But this pos-sibility scares her because she is worried that the DCIS might recur or turn into invasive cancer; she knows a neighbor who died from breast cancer after an initial diagnosis of DCIS.Your physical examination finds no abnor-malities of the breast or axilla. She has a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) of 28 and size 36C breasts without ptosis. After reviewing her mammogram, you see only a small 1-cm area of microcalcifications that would be ame-nable to a lumpectomy with needle localization of the lesion.After completing your thorough review of Debbie’s history, physical examination, and imag-ing results, you sit down with her to discuss treatment options. What do you advise?TREATMENT OPTIONS Option 1: Recommend watchful waiting with close observation.Option 2: Recommend lumpectomy with or without radiation.To aid in your decision making, each of these approaches is defended in a short essay by an expert in the field. Given your knowledge of the patient and the points made by the ex-perts, which option would you choose? Make your choice, vote, and offer your comments at .tre atment op tion 1Recommend Watchful Waiting with Close ObservationLaura Esserman, M.D., M.B.A.The first and most important message for Deb-bie is that her condition is not an emergency. The decision is about how to prevent a future breast cancer. Her recent biopsy results add in-formation about her risk. Low grade DCIS is not a cancer, and her particular diagnosis is most similar to atypia.I would explain to Debbie that breast cancer and DCIS are not one entity but a spectrum of Choose anoption andcomment onyour choice at Clinical Decisionsconditions that range from indolent to aggres-sive. She is most likely to be at risk for an indo-lent cancer, which can be treated successfully at the time of diagnosis with lumpectomy and en-docrine therapy, without radiation.1 Not surpris-ingly, evidence shows that radiation (after lum-pectomy) for DCIS does not change mortality,2 and therefore the harms of radiation outweigh the benefits.3 The question is whether she can forgo lumpectomy and just choose active surveil-lance at this time.In the majority of women with DCIS, invasive cancer will not develop. In fact, the chance of dying from breast cancer after a diagnosis of DCIS is similar to the risk in the average person without DCIS. Debbie’s risk of breast cancer extends over 10 to 20 years, not the next few months. Therefore, a preventive approach is ap-propriate, including exercising 30 minutes a day, reducing her B MI to 25 or less, and initiating endocrine risk-reducing therapy. Endocrine ther-apy has been shown, in randomized trials, to decrease the chance of getting breast cancer by at least 50%.4Debbie is anxious about doing too little but does not want a mastectomy. When a person embarks on a path that is less well traveled, join-ing a peer-reviewed clinical study can provide additional reassurance. The goal of surveillance and endocrine risk-reduction is to learn, over time, whether more treatment is needed. If she were at my institution, I would offer her partici-pation in the Cancer and Leukemia Group B (CALGB) 40903 study ( number, NCT01439711), in which a patient first under-goes magnetic resonance imaging (MRI), to rule out invasive cancer and serve as a baseline scan, and then receives aromatase-inhibitor therapy. The lesion is then followed at two consecutive 3-month intervals with repeat MRIs to look for progression. If the lesion has largely resolved, excision can be avoided. This option allows us to understand the response that Debbie, in particu-lar, has to the aromatase inhibitor. Another op-tion is participation in a registry that is opening through the Athena B reast Health Network at the University of California, in which a molecu-lar test (Oncotype DCIS) is used to reclassify low- or intermediate-grade DCIS as indolent le-sions of epithelial origin (IDLEs)5 or atypical le-sions. This reclassification then allows more in-formed discussions about the range of treatment options, including observation, treatment with endocrine agents, or lumpectomy, as appropriate. Many patients like Debbie, who are anxious about their disease, can be reassured that DCIS is not an emergency. There is time to see how their situation will evolve before they have to make a decision about surgery. Participating in trials and registries helps us all develop the best options going forward. Close follow-up should minimize any risk. Debbie has plenty of time to think about what treatment she wants, and starting with observation still leaves all options open in the future.Disclosure forms provided by the author are available with the full text of this article at .From the Departments of Surgery and Radiology, University of California San Francisco, San Francisco.tre atment op tion 2Recommend Lumpectomy with or without RadiationMonica Morrow, M.D.In a healthy 54-year-old woman who is con-cerned about future breast-cancer risk, watchful waiting is not the appropriate choice. A meta-analysis of 1385 women with low- or intermedi-ate-grade DCIS diagnosed by core biopsy 6 indi-cates that the likelihood that invasive carcinoma, not present in the needle-biopsy specimen, is present in her lesion is approximately 20%. The diagnosis of invasion would change therapy, and a 20% rate of delay in diagnosis of invasive can-cer is too hight to justify avoidance of lumpec-tomy, a brief outpatient operation with minimal complications.Once the diagnosis of DCIS is certain, clear communication between surgeon and patient regarding the risks of recurrence and death is crucial, since patients with DCIS tend to greatly overestimate the risk of death from breast can-cer.7 Debbie can be reassured that there is no necessity for mastectomy, and data from clinical trials will help to inform her decision regarding radiotherapy. In the subgroup of patients in the Eastern Cooperative Oncology Group (ECOG) 5194 study with 12 years of follow-up, excision alone for low-to-intermediate-grade DCIS less than 2.5 cm in size was associated with a 14.4% incidence of local recurrence.8 In a trial specifi-Clinical Decisionscally examining the benefit of radiotherapy in patients with low-risk DCIS (defined as low-to-intermediate grade, with a lesion less than 2.5 cm in size and a margin of 3 mm or greater), the use of radiotherapy reduced 7-year local recur-rence rates from 6.7% to 0.9%9; invasive recur-rence was seen in 42% of the women in the no-radiotherapy group. Although studies have not shown a survival benefit with radiotherapy, inva-sive local recurrence is associated with an in-creased risk of death from breast cancer, and prevention of any recurrence is a major factor in the selection of therapy for many women with DCIS,10 even if it has no effect on survival.In contrast to the body of evidence regarding outcomes of DCIS treated with excision, there are essentially no data on the outcome of DCIS managed with observation. Excellent outcomes with treatment do not necessarily translate into excellent outcomes without treatment. Evidence-based shared decision making is impossible ow-ing to a lack of knowledge about the risk that invasive cancer will develop, the likelihood of growth of DCIS precluding breast-conserving surgery, and even appropriate imaging methods and intervals for follow-up. The ongoing Low Risk DCIS (LORIS) trial (Current Controlled Trials number, ISRCTN27544579) in the United King-dom (comparing surgery with active monitoring for low-risk DCIS) will provide critical data to inform this discussion. This study will random-ly assign women 46 years of age or older with screening-detected calcifications diagnosed as non–high-grade DCIS to surgery or observation with annual mammography; the end point will be the development of invasive cancer at 5 years. In the absence of such information, observation of DCIS without excision in women who are not at high risk for death from other causes cannot be considered an appropriate approach outside the setting of a clinical trial.Disclosure forms provided by the author are available with the full text of this article at .From Memorial Sloan Kettering Cancer Center, New York.1. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a ran-domised controlled trial. Lancet Oncol 2015;16:266-73.2. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast can-cer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015;1:886-96.3. Esserman L, Yau C. Rethinking the standard for ductal car-cinoma in situ treatment. JAMA Oncol 2015;1:881-3.4. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamox-ifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727-41.5. Esserman LJ, Thompson IM, Reid B, et al. Addressing over-diagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 2014;15(6):e234-42.6. Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011;260:119-28.7. van Gestel YR, Voogd AC, Vingerhoets AJ, et al. A compari-son of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer 2007;43:549-56.8. Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year re-sults from the ECOG-ACRIN E5194 study. J Clin Oncol 2015. 9. McCormick B, Winter K, Hudis C, et al. RTOG 9804: a pro-spective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 2015;33: 709-15.10. Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol 2005;23:5526-33.DOI: 10.1056/NEJMclde1512213Copyright © 2016 Massachusetts Medical Society.392。