PNPP_4264-83-9_DataSheet_MedChemExpress
- 格式:pdf
- 大小:79.36 KB
- 文档页数:1

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。

应用基于LC—TOF—MS分析的质量亏损过滤方法筛选黄芪注射液中的皂苷类成分采用液相色谱与四极杆飞行时间质谱(LC-TOF-MS)联用技术分析黄芪注射液(HI)样品,获取正负离子模式下HI中化学成分的LC-TOF-MS分析数据。
根据前期相关文献报道,建立黄芪皂苷类化合物的质量亏损过滤(MDF)方法,用于系统筛选HI中所含有的皂苷类成分。
每个筛选化合物的存在需由不少于2个准分子离子进行确认。
最后依据其母离子和子离子信息对所筛选的化合物进行初步鉴定。
最终共从HI中筛选并初步鉴定出62个皂苷类化合物,其中15个为新发现的皂苷类化合物。
鉴定结果提示,乙酰化、氢化、去氢化、甲氧基化和水化可能为HI中皂苷所涉及的主要转化反应。
该研究丰富了黄芪化学物质基础研究内容,同时也表明基于LC-TOF-MS分析的MDF方法,是一种可行且有效的中药成分系统筛选工具。
标签:黄芪注射液;黄芪皂苷;质量亏损过滤;LC-TOF-MS;转化反应[Abstract] The samples of Huangqi injection (HI)were analyzed by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC-TOF-MS),and both positive and negative ion modes were employed to obtain the LC-TOF-MS analysis information of chemical compounds in HI. Then the mass defect filtering (MDF)approach,which was developed based on the previously published articles,was utilized to rapidly screen the astragalosides from the obtained LC-TOF-MS data. Each screened astragaloside was confirmed by the presence of no less than 2 quasi-molecular ions. All the screened astragalosides were then tentatively assigned according to the parent ion and daughter ion information. Finally,a total of 62 astragalosides were screened and characterized from the HI samples,including 15 new detected ones. The identification results indicated that acetylation,hydrogenation,dehydrogenation,methoxylation and hydration might be the major conversion reactions involved in the formation of the astragalosides. The LC-TOF-MS-based MDF approach was proved to be a feasible and efficient tool to screen the chemical constituents in complex matrices such as herbal medicines.[Key words] Huangqi injections;astragalosides;mass defect filter;LC-TOF-MS;conversion reactions.明確中药中所含有的化学成分信息,是阐明中药的药效物质基础,评价中药质量和安全性的前提和基础。

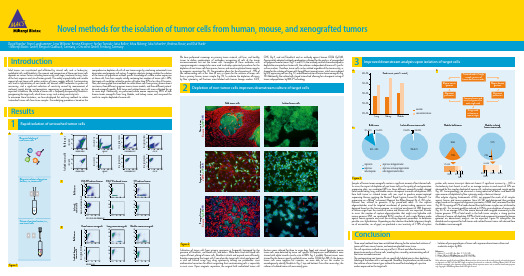
Novel methods for the isolation of tumor cells from human, mouse, and xenografted tumorsDavid Agorku¹, Anne Langhammer¹, Lena Willnow¹, Kerstin Klingner², Stefan Tomiuk¹, Jutta Kollet¹, Silvia Rüberg¹, Julia Schueler², Andreas Bosio¹, and Olaf Hardt¹1 Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, 2 Oncotest GmbH, Freiburg, GermanyIntroductionSolid tumors are vascularized and infiltrated by stromal cells such as leukocytes, endothelial cells, and fibroblasts¹. The amount and composition of those non-tumor cells depends on various factors including tumor entity and stage, treatment history, status of the host organism and site of tumor growth. The widely unpredictable and variable amount of non-tumor cells makes analyses of tumor samples difficult. Contaminating cells lead to hybridization of non-tumor cell–derived mRNA molecules to probes on microarrays, and a significant reduction of sensitivity caused by measurement of irrelevant signals during next-generation sequencing or proteome analysis can be expected. In addition, the culture of tumor cells is frequently hampered by fibroblasts overgrowing the target cells, which biases assays such as drug sensitivity tests.To overcome these limitations, we have developed fast and easy methods to isolate ‘untouched’ tumor cells from tissue samples. The underlying procedure is based on thecomprehensive depletion of cells of non-tumor origin by combining automated tissue dissociation and magnetic cell sorting. A negative selection strategy enables the isolation of the tumor cell population without specific knowledge of surface marker expression on these cells. Even from samples initially containing low numbers of tumor cells (<20%), the target cells could be isolated to purities of higher than 95% in less than 20 minutes. Here, we have applied these methods to isolate tumor cells from primary human breast carcinoma, three different syngeneic mouse tumor models, and three different patient-derived xenograft models. Bulk tumor and isolated tumor cells were cultivated for up to seven days. Additionally, we performed whole exome sequencing (WES) of bulk human tumor xenografts from lung, bladder, and kidney cancer, and compared the results to samples depleted of mouse cells.Conclusion• Three novel methods have been established allowing for the untouched isolation of tumor cells from mouse, human, and xenotransplanted tumor tissue.• The cell separation methods are easy and fast (<20 min) and allow for accurate downstream analysis of tumor cells, avoiding bias caused by contaminating cells of the tumor microenvironment.• The contaminating non-tumor cells are specifically labeled prior to their depletion. Labeling of the tumor cells is not required. Therefore, the procedures can be used for the isolation of most tumor types without the need for knowledge of a positive marker expressed on the target cells.• Isolation of pure populations of tumor cells improves downstream culture and molecular analysis by NGS.ResultsRapid isolation of untouched tumor cells1C D 326 (E p C A M )-V i o B l u e ®10¹10² C D 45-P E -V i o ® 7700010²10¹Forward scatter S i d e s c a t t e r Anti-Fibroblast-FITC GlyA-APC C D 45-P E -V i o 770CD31-PEC D 326 (E p C A M )-V i o B l u e We have performed screenings on primary tumor material, cell lines, and healthy tissues to define combinations of antibodies recognizing all cells of the tumor microenvironment but not the tumor cells. Conjugates of these antibodies with superparamagnetic nanoparticles were used to develop optimized procedures for the depletion of non-tumor cells from mouse, human, and xenotransplanted tumor samples by magnetic separation (fig. 1A). The procedure allows for the elimination of >95% of the contaminating cells in less than 20 min, as shown for the isolation of tumor cells from a primary human tumor sample (fig. 1B). To evaluate the depletion efficiency by flow cytometry, cell fractions were labeled with human lineage markers (CD31,CD45, Gly-A, and anti-Fibroblast) and an antibody against human CD326 (EpCAM).Appropriately adapted antibody combinations allowed for the analysis of xenografted or syngeneic mouse tumors (fig. 1 C and D). As the antibody cocktails were developed to deplete the unwanted non-tumor cells, the isolation is independent of tumor cell–specific surface markers. Therefore, tumor cells can be isolated regardlessof the tumor entity, as shown for the isolation of tumor cells from different mouse tumors, which were induced by GFP-expressing cell lines (fig. 1C), and different entities of human tumor xenografts (fig. 1D). Additionally, the isolated cells stayed ‘untouched’ allowing for subsequent sorting of tumor subpopulations by MACS® Technology.10³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-11Cultivation of tumor cells from primary specimens is frequently hampered by the presence of fibroblasts, red blood cells, and debris. While debris and red blood cells impair efficient plating of tumor cells, fibroblasts attach and expand more efficiently, thereby overgrowing the target cells. Even when the target cells attach and grow well, in vitro cell culture assays (e.g. drug cytotoxicity testing) are problematic since mathematical correction for effects originating from contaminating cells is impossible in most cases. Upon magnetic separation, the original bulk andisolated tumor cellfractions were cultured for three to seven days, fixed, and stained. Syngeneic mousetumor cells were detected by tumor cell–specific GFP expression and fibroblasts were stained with alpha-smooth muscle actin (α-SMA) (fig. 2, middle). Human tumors were stained for the human-specific epithelial tumor marker CD326 (EpCAM). As the human tumor cells were negative for vimentin, we were able to use this marker to unambiguously identify fibroblasts (fig. 2, top and bottom). Even after seven days, the cultures of isolated tumor cells were nearly pure.Depletion of non-tumor cells improves downstream culture of target cells2V i m e n t i n / E p C A M / D A P IH u m a n t u m o r Bulk tumor cellsIsolated tumor cellsS y n g e n e i c m o u s e t u m o r α-S M A / e G F P / D A P IX e n o g r a f t t u m o r V i m e n t i n / E p C A M / D A P IFigure 2References1. DeRose, Y.S. et al. (2011) Nat. Med. 17: 1514–1520.2. Bolger, A.M. et al. (2014) Bioinformatics 30: 2114–2120.3. Li, H. and Durbin, R. (2009) Bioinformatics 25: 1754–1760.Unless otherwise specifically indicated, Miltenyi Biotec products and services are for research use only and not for therapeutic or diagnostic use. MACS and the MACS logo are registered trademarks or trademarks of Miltenyi Biotec GmbH. All other trademarks mentioned in this document are the property of their respective owners and are used for identification purposes only. Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.Samples of human tumor xenografts contain a significant amount of host-derived cells. To assess the impact of depletion of non-tumor cells on the quality of next-generation sequencing data, we conducted WES on three different xenograft models derived from human kidney, lung, and bladder cancer subsequent to mouse cell depletion. DNA from bulk tumor or isolated tumor cells was used to produce exome-captured sequencing libraries applying the Nextera® Rapid Capture Exome K it (Illumina®). For sequencing on a MiSeq® instrument (Illumina) the MiSeq Reagent K it v3 (150 cycles, Illumina) was utilized to generate 75-bp paired-end reads. As the capture oligonucleotides used for targeted enrichment of protein-coding sequences were designed based on the human genome, an initial pre-enrichment of DNA fragments of human origin from the mixture of mouse and human cells was expected. In order to assess the number of capture oligonucleotides that might cross-hybridize with mouse genomic DNA, we conducted BLAST searches of each single Nextera probe against mouse genome and used the resulting alignment parameters to determine possible cross-hybridization. Depending on the selection thresholds (alignment length, no. of mismatches, no. of gaps), we predicted a cross-reactivity of 5–10% of captureprobes with mouse transcripts (data not shown). A significant increase (p < 0.05) in clusterdensity (not shown) as well as an average increase in read counts of 33% was observed for the samples depleted of mouse cells, indicating improved sample quality (fig. 3A). Correspondingly, we observed a strong reduction of debris and dead cells upon mouse cell depletion by flow cytometry analysis (data not shown).After adapter clipping (trimmomatic v0.32²), we mapped the reads of all samples against human and mouse genomes (bwa v0.7.12³) and determined their putative origin based on the respective alignment parameters (LINUX shell, command-line Perl) (fig. 3B). An average of 12% of reads derived from bulk tumor samples was attributed to mouse cells. This amount could be reduced to 0.3% by prior depletion of mouse cells (fig. 3C). As on average 15% of the mouse-derived reads mapped erroneously to the human genome (1.9% of total reads) in the bulk tumor samples, a strong positive influence of mouse cell depletion (0.04% of total reads erroneously mapped to human genome) on downstream analyses can be expected. Figure 3C exemplifies the detailed read assignment for bulk tumor and isolated human tumor cells derived from the bladder cancer xenograft.Improved downstream analysis upon isolation of target cells3。

三重串联四极杆液质联用仪品牌:WA TERS1、设备名称:液相色谱串联四极杆质谱联用仪2、主要用途:用于多种农、兽药残留、生物毒素残留、食品添加剂的检测,动物及动植物源食品的残留监控工作,特别是对检测结果为阳性的样品,可进行结果确证,并且可以对预期要开展的检测项目进行检测技术储备,以加强对出口食品、农产品安全卫生质量的监管和控制。
3、工件条件:工作电压:230V±10%温度:18-250C湿度:350-85%4、技术指标:1)超高效液相色谱系统技术要求1.1二元或四元泵*1.1.1压力:0-15,000 psi1.1.2流量:0.001-2.000ml/min, 以0.001ml/min 为增量1.1.3精度:≤0.075%RSD1.1.4流速准确度:±1.0%1.1.5延迟体积:<120μL1.1.6溶剂数量:4种1.1.7设定范围:室温上5 – 60 °C,1°C 步距1.1.8温度控制精度:±0.1oC温度1.2自动进样器1.2.1精度:< 0.5%1.2.2样品交叉污染度:<0.005%1.2.3进样准确度:±1 μL1.2.4进样体积:0.1 ~50μl1.2.5进样线性度: > 0.9991.3在线真空脱气机1.4二极管阵列检测器1.4.1波长范围:190-800nm1.4.2二极管个数:1024个1.4.3光学分辨率:1.2nm1.4.4狭缝宽度:1,2,4,8,16nm1.4.5实时信号:同时输出8个实时信号1.4.6波长精度:1nm1.4.7基线噪音:±1.0 x 10-5 AU,在254nm和750nm(1mL/min甲醇)1.4.8基线漂移: 2 x 10-3AU/hr , 在254nm(1mL/min甲醇)1.4.9数据采集率:〉80Hz2)质谱部分指标*2.1质量范围m/z: MS1及MS2:30-3,000amu2.2分辨率:?2.5M2.3质量数稳定性:≤0.1 amu /8H2.4电喷雾灵敏度指标(相同绝对量,不同浓度下的灵敏度):1pg利血平,m/z 609-195,信噪比≥500:12.5ESI负离子:2.5pg氯霉素,m/z321-152,信噪比≥100:12.6正、负离子采集切换速率≤20 ms2.7MRM扫描时间≤5 ms,一个采集通道可MRM定量数≥502.8扫描速率:?5000amu/秒2.9动态范围:?4X1062.10质量精度:≤0.1 amu2.11离子源:大气压离子源是双正交设计,而且离子源和质谱间有隔断阀,锥孔有N2保护气,可容忍不挥发性的缓冲盐。


Endoproteinase Lys-C from Lysobacter enzymogenessuitable for Protein Sequencing Catalog Number P3428Storage Temperature 2–8 °CTECHNICAL BULLETINCAS RN 72561-05-8EC 3.4.21.50Synonym: Lys-CProduct DescriptionEndoproteinase Lys-C from Lysobacter enzymogenes is a serine endoprotease, which hydrolyzes peptide bonds at the carboxyl side of lysyl residues.1-6Lys-Proand Lys-Glu bonds are also cleaved.5Some minornon-specific cleavage has been reported.2,5The protease readily cleaves at aminoethylcysteineresidues.6Endoproteinase Lys-C is HPLC purified, resulting in a product that is suitable for protein sequencing. In 100 mM NH 4HCO 3, pH 8.5, or 100 mM Tris HCl,pH 8.5, Lys-C specifically cleaves peptide bonds at the carboxyl side of lysine.It is widely used for proteinsequencing work due to this highly specific cleavage of peptides resulting in a limited number of fragments.1-6Average molecular mass:127.96 kDa Optimal pH:1∼8.5Vial content: 5 µg of lyophilized Lys-C containing HEPES, EDTA, and raffinose.Precautions and DisclaimerThis product is for R&D use only, not for drug,household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.Preparation InstructionsReconstitute the lyophilized product in 50 µl of water.The protease will be in a solution containing 50 mM HEPES, pH 8.0, 10 mM EDTA, and 5 mg/ml of raffinose.Storage/StabilityStore the lyophilized powder desiccated at 2–8 °C. The hygroscopic nature of the lyophilized powder may make it appear wet. The activity and suitability of the enzyme is not affected.After reconstitution in water, frozen aliquots can bestored for several weeks.4ProcedureFor peptide or protein digestion, a ratio between 1/20 and 1/100 (w/w) of enzyme to substrate is recommended.1.Dissolve the peptide or protein to be digested in100 mM NH 4HCO 3, pH 8.5, or 100 mM Tris HCl, pH 8.5.2.Recommended incubation time is between 2 and18 hours at 37 °C depending on the enzyme/ substrate ratio. Notes: Self-digestion of may occur if temperatures >37 °C are used.Lys-C retains most of its activity in 2.0 M urea or 0.1% SDS.2,3,6A peptide such as melittin or the oxidizedB chain of insulin should be run as a control for all experiments. Endoproteinase Lys-C may also be used for in-gel digestions of proteins.7-11ResultsThe suitability of this product is demonstrated by the digestion of melittin (Catalog Number M4171; see Figure 1). The sequence of melittin is:GIGAVLKVLTTGLPALISWIKRKRQQFigure 1.Suitability Assay Of Lys-CMelittin (100 µg) was digested with 5 µg of Lys-C for 18 hours at 37 °C in 100 µl of 100 mM NH 4HCO 3, pH 8.5. A 20 µg aliquot was separated on a Discovery ®C 18column (25 cm ×4.6 mm,5 µm, Catalog Number 504971) using a 20 minute linear gradient from 5–50% B at 0.7 ml/min, and was detected in the UV at 214 nm and by mass spectrometry. Solvent A: 0.1% (v/v) TFA in waterSolvent B: 0.08% (v/v) TFA in acetonitrileThe Lys-C peptide fragments were identified as follows:Retention Time (min)Mass (Da)Fragment5.79429.3Arg(24)-Gln(26)6.08302.3Arg(22)-Lys(23)15.36656.4Gly(1)-Lys(7)22.711,512.0Val(8)-Lys(21)The two short hydrophilic peptides (retention times of 5.79 and 6.08) co-elute with the unbound buffer salts in the injection peak. The retention times for these two peptides were determined by searching for their expected masses.During the 18 hour digestion only the expected peptides were generated with no indication of other majorproteolytic activity. Under the experimental conditions the cleavage of the test peptide was complete in less than one hour.References1.Smith, B.J., Methods in Molecular Biology, Volume3, New Protein Techniques, Humana Press (New Jersey: 1988), p. 57.2.Aitken, A. et al., Protein Sequencing: A PracticalApproach, IRL Press (Oxford, UK: 1989), p. 43.3.Burdon, R.H., and Knippenberg, P.H., (eds.),Laboratory Techniques in Biochemistry and Molecular Biology: Sequencing of Proteins and Peptides,Volume 9, Elsevier (New York, NY: 1989), p. 73.4.Stone, K.L. et al., A Practical Guide to Protein andPeptide Purification for Microsequencing, Academic Press, Inc. (New York, NY: 1989), p. 31.5.Jekel, P.A. et al., Anal. Biochem., 134, 347-354(1983).6.Tarr,G.E. Methods of ProteinMicrocharacterization: A Practical Approach, Humana Press (New Jersey: 1986), p. 168.7.Xu, S-Q. et al., J. Biol. Chem., 273, 20078-20083,(1998).8.Jeno, P. et al., Anal. Biochem., 224, 75-82 (1995).9.O’Connell, K.L., and Stults, J.T.,Electrophoresis,18, 349-359 (1997).10.Patterson, S.D., Electrophoresis, 16, 1104-1114(1995).11.Fountoulakis, M. et al., Electrophoresis, 19, 1819-1827 (1998).Related ProductsHPLC Purified Products:Product Name Catalog Numberα-ChymotrypsinC6423Endoproteinase Arg-C P6056Endoproteinase Asp-N P3303Endoproteinase Glu-C P6181Endoproteinase Lys-C P3428Leucine aminopeptidase L9776TrypsinT8658Insulin Chain B, Oxidized I1764α-Melanocyte Stimulating HormoneM4135Discovery is a registered trademark of Sigma-Aldrich Co. LLC.GY,LKB,MAM 05/14-1©2014 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for theirparticular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.Time (min)A b s o r b a n c e , 214n m (m A u )0400800120016005101520255.796.0815.3622.71。

ABSTRACTProducts formed from defined oligodeoxyribonucleotide tetramers (oligonucleotides) by depurination at pH 5.0 and 900C followed by chain breakage at the resulting apurinic sites (AP sites) were assigned by reversed phase HPLC. Through kinetic analysis, rate constants of depurination and subsequent chain breakage reactions were measured. Depurination of the oligonucleotides with purine bases locating at the terminal positions was several times faster than those with purines at the internal ones. The pKa values for the N7 of the G residues and the activation energies of the depurination were essentially independent of the position of the bases. The frequency factor was found to be responsible for the observed difference of the depurination rates. In contrast, the chain breakage by β-elimination was several times faster for the AP sites formed at the internal positions than those at the 5'-terminal positions. It is suggested that an electron withdrawing phosphate group attached to the 5'-side of an AP site facilitates the chain cleavage.摘要pH为5.0温度为900C条件下,由于无嘌呤位点(AP位点)处的断链而造成的特定寡核苷酸[由少于20个碱基组成的短链核苷酸](dna或rna)四聚体,其组成成分由反相高效液相色谱法[由非极性固定相和弱极性流动相所组成的液相色谱体系]所分别开来。

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:M3814 is a potent and selective inhibitor of DNA-dependent Protein Kinase (DNA-PK ).IC50 & Target: DNA-PK [1]In Vivo: In combination with IR, M3814 shows efficacy in all of the 6 mouse models of human cancer. In all models, a dose of 2 Gy administered daily for 1 week in combination with M3814 induces statically significant tumor growth inhibition compare to IR alone.M3814, alone or in combination with IR, does not induce significant weight loss or visual signs of toxicity in the mice in any study [1].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration:[1]The efficacy of M3814 in combination with IR is evaluated in 6 human xenograft models (HCT116, FaDu,NCI-H460, A549, Capan-1, BxPC3) in mice representing 4 different cancer types (colon, head and neck, lung, and pancreas). Tumor cells are injected s.c. into nude mice , and treatment starts when palpable tumors are established (~100 to 200 mm 3 ). M3814 is given orally at different doses (25 to 300 mg/kg ) 10 min prior to IR. IR is applied using a radiation therapy device for small rodents calibrated to deliver 2 Gy. Autophosphorylation of DNA-PK (serine 2056 ) in FaDu tumor lysates is measured by immunoassay to assess pharmacological inhibition by M3814[1].References:[1]. L. Damstrup, et al. M3814, a DNA-dependent Protein Kinase Inhibitor (DNA-PKi), Potentiates the Effect of Ionizing Radiation (IR) in Xenotransplanted Tumors in Nude Mice. IJROBP. 2016; 94, 940-941.[2]. Frank T. Zenke, et al. Abstract 1658: M3814, a novel investigational DNA-PK inhibitor: enhancing the effect of fractionated radiotherapy leading to complete regression of tumors in mice. AACR; Cancer Res 2016;76(14 Suppl):Abstract nr 1658.Product Name:M3814Cat. No.:HY-101570CAS No.:1637542-33-6Molecular Formula:C 24H 21ClFN 5O 3Molecular Weight:481.91Target:DNA-PK Pathway:Cell Cycle/DNA Damage; PI3K/Akt/mTOR Solubility:DMSO: 250 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。

Seppro®IgY 14 LC10 ColumnCatalog Number SEP040Storage Temperature 2–8 °CTECHNICAL BULLETINProduct DescriptionThe Seppro®IgY 14 Liquid Chromatography 10 (LC10) Column is based on avian antibody (IgY)-antigen interactions and optimized buffers for sample loading, washing, eluting, and column regeneration. The column is specifically designed to remove fourteen highly abundant proteins from human fluids such as serum or plasma. The following proteins are depleted in a single step:Albumin IgGα1-Antitrypsin IgAIgM TransferrinHaptoglobin α2-Macroglobulin Fibrinogen Complement C3α1-Acid Glycoprotein (Orosomucoid)HDL (Apolipoproteins A-I and A-II)LDL (mainly Apolipoprotein B)The targeted highly abundant proteins are simultaneously removed by the immobilized specific IgYs when crude biological samples are passed through the column.Selective immunodepletion provides an enriched pool of low abundance proteins for downstream proteomic analyses. Specific removal of these fourteen highly abundant proteins depletes ∼95% of the total protein mass from human serum or plasma. The low abundance proteins in the flow-through fractions can then be studied. Removal of highly abundant proteins enables improved resolution and dynamic range for one dimensional electrophoresis (1DGE), two dimensional (2DGE) electrophoresis, and liquid chromatography/ mass spectrometry (LC/MS). The collected flow-through fractions may need to be concentrated dependent upon the downstream application.Characteristics of the IgY 14 LC10 ColumnSize: 12.7 ×79.0 mm(10 ml bed volume)Capacity: 10.0 mg of total protein or ∼143 µl of human plasma based on an average protein concentration of 70 mg protein/ml.Note: If the protein concentration of the sample is unknown and the total serum protein level is potentially elevated, a reduction of the serum load to 100 µl is recommended for initial study to avoid potential abundant protein bleed through.Total protein mass removal: ∼95%Targeted depletion efficiency: 95% (average) Maximum operation pressure: 350 psi (21 bars) Antibody-modified resin only withstands 100 psi Flow rate: 0.5–2.0 ml/minuteOperating temperature: 18–25 °CShipping Buffer: 1×Dilution Buffer with 0.02% sodium azideColumn body materials: Polycarbonate column cylinder, Polyethylene frit, Tefzel®caps, Buna-N-rubberO-rings, Delrin®nut fittings, ETFE ferrules, andPTFE PFA tubing.Usage: Column may be used 100 times.2ComponentsSeppro IgY 14 LC10 Column 1 each (Catalog Number S5074)10×Dilution Buffer 3 ×200 ml Tris-Buffered Saline (TBS) -100 mMTris-HCl with 1.5 M NaCl, pH 7.4(Catalog Number S4199)10×Stripping Buffer 3 ×200 ml1 M Glycine, pH 2.5(Catalog Number S4324)10×Neutralization Buffer 3 ×80 ml1 M Tris-HCl, pH 8.0(Catalog Number S4449)Corning®Spin-X®Centrifuge Tube Filters 1 pack0.45 µm, pack of 100(Catalog Number CLS8163)Precautions and DisclaimerThis product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Specimen collection needs to utilize universal precautions.Preparation InstructionsPreparation of 1×concentration buffers -Separately dilute the three 10×buffers (Dilution, Stripping, and Neutralization Buffers) 10-fold with water. If precipitation occurs in the 10×buffers, allow the bottle to warm to room temperature and mix untilcompletely dissolved prior to use. Do not dilute all of the 10×Neutralization Buffer, save a volume of the 10×neutralization buffer for neutralization of eluted bound proteins if analysis of bound proteins is desired. Sample Preparation -It is not recommended to load unfiltered serum directly onto the column. Human serum samples should be diluted 5-fold with 1×Dilution Buffer. Samples may contain particulate materials, which can be removed with a 0.45 µm spin filter, centrifuge for 1 minute at 9,000 ×g.Storage/StabilityStore the column at 2–8 °C. After use, equilibrate the column with 1×Dilution Buffer containing 0.02% sodium azide and store the column at 2–8 °C with the end-caps tightly sealed. Do Not Freeze the column.ProcedureNote: Always use the three 1×buffers as the mobile phases for the LC procedure. Adjust the LC procedure appropriately for the instrumentation being used. Do not expose the column to solvents other than the three 1×buffers. Do not expose the column to organic solvents (like alcohols, acetonitrile, etc.), strong oxidizers, acids, or reducing agents and other protein denaturing agents (urea).1.Set up the three 1×buffers as the only mobilephases.2.Purge lines with the three 1×buffers and run the1×Dilution Buffer at 2 ml/minute without a columnto check the system back pressure.Note: The maximum operation pressure includesthe pressure introduced by the column and thesystem backpressure from the instrument. Usually, the pressure introduced by the column is less than50 psi. It is important to first check the systembackpressure of the instrument before using thiscolumn. If the system backpressure is more than300 psi, use tubing with a larger I.D.or change the flow-cell to tubing with a larger I.D. to reduce thesystem backpressure.3.Attach the column to chromatography instrument(see Appendix) and equilibrate it with1×DilutionBuffer for 20 minutes at a flow rate of 2.0 ml/minute to obtain a flat baseline.4.Set up a LC timetable (see Table 1) and run twomethod blanks by injecting 1,250µl of1×DilutionBuffer.Note: Adjust LC timetable based on instrumentation available, if necessary.5.Inject 1,250 µl of the diluted and filtered serum (seeSample Preparation), start with a flow rate of0.5 ml/minute for 30 minutes, wash the column at aflow rate of 2.0 ml/minute for 5 minutes, collectflow-through fraction, and store collected fractionsat –70 °C if not analyzed immediately.Note: Due to high salt concentration in the1×Dilution Buffer, buffer exchange of the flow-through fractions to a volatile buffer (for example,ammonium bicarbonate) is recommended prior tolyophilization.36.Elute bound proteins from the column with1×Stripping Buffer at a flow rate of 2.0 ml/minutefor 15 minutes and neutralize the eluted fractionswith 0.1×fraction volume of 10×Neutralizing Buffer.Note: Do not expose the column to the 1×Stripping Buffer for more than 20 minutes.7.Neutralize the column with 1×Neutralizing Buffer ata flow rate of 2.0 ml/minute for 10minutes.8.Re-equilibrate the column with 1×Dilution Buffer foran additional 10 minutes at a flow rate of2.0 ml/minute. The re-equilibrated column may bestored with 1×Dilution Buffer with 0.02% sodiumazide at 2–8 °C. Do Not Freeze the column.Table 1.Timetable for IgY 14 LC10 columnDetector Model: 166.ResultsFigure 1.Typical Depletion ChromatogramPeaks A & B aredepleted fractions.Peak C is boundfraction.ABC4AppendixTips for fitting column to most chromatography instruments1.Adapting the column (M6 fitting) to most HPLCsystems• Option 1: female M6 to male 10-32 one-piece adapter (Catalog Number 55069) -Most HPLCinstruments use 10-32 fittings with 1/16″tubing,so an adapter (one-piece fitting) can be used toconnect the column to the instrument. Thisinexpensive approach uses an adapter, a one-piece fitting with female M6 threading at oneend, to accept the male M6 fitting on the tubingconnected to the column, and 10-32 malethreading on the other end, to fit into thedetector or injector.Note: Use of this adapter will require removal ofthe fitting on the injector or detector.• Option 2: female M6/male 10-32 fitting tofemale 10-32/female 10-32 fitting two-pieceadapter (Catalog Number55068) –A two-pieceadapter can be used without changing thedetector or injector fittings on the HPLC. Again,the inexpensive approach is a two-pieceadapter. The female M6 end of the female/malefitting accepts the male M6 fitting from thecolumn, the male 10-32 end fits into one end ofthe female/ female 10-32 fitting. The other endof the female/female fitting accepts the 10-32fitting on the HPLC tubing.2.Adapting the column (M6 fitting) to non-metricmedium/low pressure liquid chromatographysystems• Option 1: female 1/4-28 to male 10-32 one-piece adapter (Catalog Number 55071) plus10-32 to female M6 two-piece adapter (CatalogNumber 55068) -Many medium/low pressuresystems use 1/4-28 fittings. An one-pieceadapter along with a two-piece adapter can beused to connect the column with M6 fittings tothese instruments. This method requires havingtubing with 1/4-28 fittings on the injector anddetector. These fittings will thread into thefemale 1/4-28 to male 10-32 fitting (CatalogNumber 55071). The male 10-32 will tread intothe two-piece adapter (Catalog Number 55068)that will then join to the M6 fitting.• Option 2: female 1/4-28 to female M6 one-piece adapter(Catalog Number 59259-U) -Like Option 1, this option requires the tubing onthe injector and detector already have male1/4-28 fittings. The male 1/4-28 fitting willthread into the adapter that will then join it tothe male M6 fitting.Note:Be sure to order two of these parts, onefor each end of the column.Troubleshooting GuideHigh backpressure -Clogged inlet frits may result in high backpressure, distorted peak shape, and diminished column lifetime. To prevent these problems, remove particulates from samples with a spin filter before loading.No bound fraction peak -Bound proteins can only be removed from the column by eluting with 1×Stripping Buffer. Check LC timetable to ensure enough column exposure time to the 1×Stripping Buffer for complete removal of bound proteins.Abnormal peak height -∼95% of serum/plasma proteins will be removed as the bound fraction. The peak height of the bound fraction is expected to be much greater than that of the flow-through fraction. If this order is reversed, two possibilities may be checked:• Column may not have been regenerated properly after previous use,resulting in lost capacity. Tocorrect this, elute bound proteins with 2 additionalcolumn volumes of 1×Stripping Buffer and thenneutralize and re-equilibrate the column with1×Neutralizing Buffer and 1×Dilution Buffer.• Check for signs of biological growth in the buffer reservoirs. Replace with fresh buffers for optimized column performance.Seppro is a registered trademark of Sigma-Aldrich®Biotechnology LP and Sigma-Aldrich Co.Corning and Spin-X are registered trademarks of Corning, Inc.Delrin and Tefzel are registered trademarks of E.I. du Pont de Nemours & Co., Inc.TD,KR,DEC,MAM,MJM 11/14-2©2014 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.。

Your Quality, Our Assurance Flow-Through Cell Dissolution Tester Ophthalmic SuspensionsMedical DevicesNanoparticlesIVIVC StudiesInjectablesGranules2Flow-Through Cell Dissolution Tester (USP Apparatus 4)Flow-Through cell dissolution tester is widely recommended for poorly soluble, modified release and extended release dosage forms. With the evolution of new drug delivery platforms, USP apparatus 4 is best recommended for studying the dissolution profile of solid, liquid, oral, non-oral dosage forms and other medical devices such as stents, implants etc. As this apparatus offers highly flexible configurations, ability to work in variety of solubility conditions, different types of cells and positioning of the dosage form, hydrodynamics, sink conditions and flow rates, USP apparatus 4 will continue to evolve to meet the changing needs of today’s dissolution and drug release testing.The USP apparatus 4 comprises of a media reservoir to hold the dissolution media, a pump that forces the media upwards through a vertically positioned flow-through cell that holds the dosage form and a water bath to maintain the cell temperature.The test sample is placed in a vertically positioned flow-through cell through which the media is pumped at a desired flow rate and temperature. The eluate is filtered at top of the cell and is then collected either manually or by a sample collector. The samples are further analyzed using suitable analytical techniques to calculate the percent drug release.The pump unit is responsible for ensuring the most critical parameter of USP apparatus 4 i.e 1. 2. The flow rate must be constant throughout the test, even in cases of back pressure created by the filters. . The USP recommends the optimum 0media temperature should be 37C.Flow rate of the media Temperature ± 0.5 as per USPThe USP regulation recommends that the flow profile should be sinusoidal with pulsation of 120 ± 10 pulses/min ŸUSP apparatus 4 is the ideal choice for poorly soluble drugsŸUSP apparatus 4 is the best method of choice for large media volume dissolution, in order to achieve infinite sink condition ŸFor IVIVC studies, automated media changeovers can be easily achieved for solid as well liquid dosage forms ŸFlow rates can be easily changed to allow ‘accelerated’ test studies ŸMany challenges such as tablet floating, sticking etc. are eliminatedMethodologySystem Specifications as per USP RecommendationSystem ComponentsWhy choose USP Apparatus 4?Heat ExchangerCoilPiston PumpSampleCollectorMedia SelectorMediaReserviorsWaste1234Flow Through Cell Open LoopoCDifferentialtdmC(t) =edtQ((Although USP apparatus 1, 2 and 3 can be used for studying the dissolution profile of poorly soluble drugs, but they fail to offer the optimal sink conditions required. Whereas, USP apparatus 4h as a lways b een l inked t o "optimal s ink c onditions" a s i t o ffers t he f lexibility i n t erms of media volume required. In an "open loop" configuration, there is a continuous flow of fresh media across the dosage form and hence, the total media volume used can be infinite. This means, that the influence of poor sink conditions on the test can be avoided by using larger volumes of media without the need for solubilizing agents. Samples can be collected as a fraction over a timed interval and analyzed using suitable analytical technique. The total amount of media passing through the dosage form is determined by the flow rate. In an open loop configuration, results are calculated as a differential curve or rate of drug release over the time.In an open loop configuration, EFD-07 can be integrated with a sample collector with splitter that enables sample collection for 14 sampling intervals (21 optional) with 75 mL of sample collection volume. Sample collector with splitter, automatically splits the sample volume into collection and waste depending upon the sample volume required.Media Selector Automated media changeover :Unlike the USP apparatus 1 and 2 involving media changeovers, where physical removal of the dosage and change to a new media is cumbersome and tedious, USP apparatus 4-open loop configuration facilitates easy media changeover at predefined time intervals. The flow-through method is the only method that allows for a media changeovers of suspensions and powders. This feature is useful while performing IVIVC studies, where the dosage form naturally passes through different pH of the digestive tract within sink conditions. It is also useful for enteric coated products, modified and extended release drug products. Using EFD-07 media selector, 4 different media can be automatically drawn from different sources at predetermined intervals.Sample Collector with SplitterTMD’light technologyfor audio and visual indication of instrumentstatus from anywhere in the laboratoryMovement of arm to :Sample positionWaste tray / home positionPower onNeedle diving up / downCommunication with dissolution testerError due to :Absence of waste trayAbsence of sample collector traySample collector hood ajarCompletely covered to prevent contamination and air drafts which causeexcess evaporation of the collected samplesOpen Loop Configuration34oC Cumulativedm C (t) =e V htThe closed loop configuration of USP apparatus 4 is similar to USP apparatus 1 and 2 where a fixed volume of media is recirculated through the dosage form. Samples can be withdrawn from the media reservoir at predetermined time intervals either manually or by a syringe system. The samples can be analyzed using suitable analytical technique. In case of closed loop, result of percent drug release dissolved is expressed as a cumulative curve. Closed loop configuration is ideal for dosage forms where solubility and sink conditions are optimal in a limited media volume range from 40 mL to 4 L. For low dose formulations such as drug eluting stents, implants, coated medical devices, injectable and microspheres; closed loop configuration has been utilized to fulfill lower media volume testing. The problems such as tablet sticking, floating, coning or dead zones seen in USP 1 and 2 as well as sampling issues and sample introduction effects are eliminated using the USP apparatus 4.Off-line sample collection for the closed loop configuration is available with EFD-07 connected to a sample collector . Syringes pull the desired volume from the media reservoir and dispense them either into glass tubes (20 mL or 10 mL) or capped HPLC vials. The system can be programmed to collect the sample volumes at predefined time points. The syringe system also offers the facility for auto-replacement and dilution with fresh media. The sample collector enables sample collection for 24 sampling intervals. Automatic sample collector adapts to 5 different types of trays.Sample Collector and Syringe PumpClosed LoopEFD-07 can also be integrated with a media manager to maintain the desired media temperature and provide continuous stirring of media in the media reservoir throughout the test.Closed Loop ConfigurationHeat Exchanger CoilPiston PumpMedia ReservoirAutomated SamplingManual SamplingFlow Through CellSyringe PumpCombination tray (with dilution option)HPLC vialsGlass tubesHPLC and Glass tubes (2 Types)ŸHPLC vials 1.5 mL (8 x 7) + Glass tubes 20 mL (8 x 7)*Customized trays availableSample Collector Tray (ESC-08Dx)* Ÿ1.5 mL (24 x 7)Ÿ20 mL (16 x 7)5ComplianceTouch ScreenX7°CIndividual Cell Temp. MonitorProgrammable Protocols999Print Report LAN Audio OutputT e s t Rep o r tAutomated Offline SamplingQuality by DesignQbD ApplicationsUSP apparatus 4 facilitates dissolution testing of:ŸTablets ŸCapsulesŸPowder / granules / API's / bead formulation ŸInjectable suspensionsŸSuppositories / soft gelatin capsules ŸMicrospheres / liposomes / nanoparticles ŸInhaler drugsŸDrug eluting stents / implants ŸOintments / creams / gels ŸOphthalmic lensesŸCompliance with USP , Ph. Eur. JP and BP Ÿ7 cell dissolution tester ŸValveless ceramic pump headsŸAutomatic flow rate adjustment for individual cellsŸIndividual cell temperature monitoringŸMedia selector for easy media change (optional) for open loopŸProgram support for gravimetric flow validation and calibration of individual pumpsŸIsolated water circulating pump for precise temperature control of the water bath and to reduce vibrationsŸUser friendly intuitive 7” touch screen interfaceŸFlow rates can be adjusted from 2 mL/min to 32 mL/minŸ999 programmable protocolsŸSaves upto 100 calibration and validationreportsŸfor system and sample collector status. For eg. Connected Run ErrorTest protocol can also be printed using in-bult thermal printer for better longetivity of data (optional)TMD’lighttechnology Ÿn n n E F D -07Features of EFD-07USP, Ph.Eur.,JP6As USP apparatus 4 has wide range of applications, several flow-through cells have been developed and optimized according to the different dosage form like tablets, suspensions, stents, suppository etc. EFD-07 is capable of employing the method to accommodate most dosage forms.1.12 mm cell : This cell is described as a small cell for tablets and capsules in theUSP , Ph. Eur. and JP . As per the specifications, a detachable tablet holder is also available. This cell can also be used for suspensions, injectables, small medical devices and stents.2.22.6 mm cell : This is the most widely used cell of all flow-through cells. It isdescribed as a large cell for tablets and capsules in the USP , Ph. Eur. and JP . As per the specifications, a detachable tablet holder is also available. It can also be used for larger doses of suspensions and microspheres. There are a variety of holders developed for holding different dosage forms in this cell.3.Cell for powders and granules : This cell is described in the Ph. Eur. and isbased on the 12 mm cell. It is used to determine the dissolution rate of pure solid substances (API characterization), active substances in preparations used as powders, granules and bead formulations.4.Dialysis adapter in 22.6 mm cell : This cell is based on the 22.6 mm cell and isused to study the dissolution profile of nanoparticles, microspheres, micro-suspensions and injectables etc. A dialysis adapter along with dialysis membrane inside the cell allows testing on these dosage forms. Adapter to accommodate 1 mL Float-A-Lyzer is also available.5.Cell for suppositories and soft gelatin capsules : This cell is described in thePh. Eur. and has a special two chambered design which blocks the lipidic excipients from the suppository/soft gelatin capsules and allows only the dissolution media to pass up to the filter.6.Aerosol/Inhaler cell : This cell is manufactured in stainless steel and isspecially designed to study the elution rate of inhaler drugs.1 : 12 mm cell2 : 22.6 mm cell3 : Cell for powders and granules4 : Dialysis adapter in 22.6 mm cell5 : Cell for suppositories and soft gelatin capsules6 : Aerosol/Inhaler cell7.Cell for drug eluting stents: This cell is manufactured in PTF and is used for medical devices like drug elutingstents. The inner diameter can be customized to fit the medical device accordingly. 8. Cell for large medical devices : This cell has a maximum length of 80 mm and is designed to hold longer medical devices.9.Cell for implants: This cell has a small chamber to house the dosage and is used for smaller implants.Flow-Through cells for different dosage forms710. Customized flow-through cells :Using the above cells as the main models, specific holders have also been designed to hold other dosage forms. Customization can be based on the dosage form, media, inner diameters, cell length and holding devices etc. • Holder for creams and gels : An inserted cup facilitates testing on ointments, gels and creams using apermeation membrane. This modification is based on 22.6 mm cell.• Holder for ophthalmic lens : This modification is based on 22.6 mm cell and has an inverted holder thatallows testing on ophthalmic lenses coated with drugs.For intended use of hydroalcoholic media, specially designed PEEK cells are also available•Types of flow :s :There are 2 types of hydrodynamic flow within a flow-through cell viz. a laminar flow and turbulent flow. Laminar flow is achieved by filling the flow-through cell with a 5 mm ruby bead bottom of the cell followed by a layer of 1 mm glass beads as described in the USP . The laminar flow is more controlled as it crosses the dosage form in unidirectional flow. The turbulent type of flow is obtained by placing only the ruby bead in the flow-through cell. The turbulent flow is more beneficial for dosage forms that require a higher agitation rate to release its actives.Experimental ConditionsTurbulent FlowŸSample filtration :Filtration occurs at the top of the flow-through cell with a filter insert of standard filter size of 25 mm. Different types of filters with variety of pore sizes can be used depending on the dosage form. In some cases, multiple filters can be used from larger to smaller pore size. The use of glass wool in the filter section is recommended for dosage forms with highly insoluble or oily particulates .ŸDosage positioning :Solid dosage form: Tablet can be simply placed in the cell on the layer of glass beads or positioned uniformly on atablet clip holder or directly on the ruby bead (turbulent flow). This factor can eliminate problems such as tablet sticking, swelling and floating as seen in other conventional dissolution methods.Suspension :Liquid samples can be placed directly on layer of glass beads or sandwiched uniformly between single or multiple layers of glass beads. This ensures repeatability, reproducibility and reliability of results.Powder :Using the powder granule cell, the powder is simply filled into the cell without any compression or compaction. The filter insert is placed on either side of the cell.Laminar Flowaccepts no liability for any errors and reserves the right to alter specifications without noticeDecember, 2015ELECTROLABSales &Service WorldwideEFD-07M.S. : 1410100110 Volts 230 Volts M.S. : 1420100ModelProduct code Utilize ELECTROLAB ’s dedicated Dissolution Application Lab for confidential MethodDevelopment and Transposition to optimize parameters for NDDS。

PFTBA Calibration Compound (FC-43), Part Number 392035300*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::化学名:全氟三丁胺供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818PFTBA Calibration Compound (FC-43), Part Number 392035300化学品的推荐用途和限制用途392035300部件号:物质用途:供分析化学实验室使用的试剂和标准2 ml(毫升) 安瓿安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:PFTBA 校正化合物 (FC-43),部件号 392035300有关环境保护措施,请参阅第 12 节。
物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述液体。
[吸湿的。
]无色。
无气味的。
不易生物降解。
H315 - 造成皮肤刺激。
H319 - 造成严重眼刺激。
H335 - 可能造成呼吸道刺激。
H413 - 可能对水生生物造成长期持续有害影响。
物理状态:颜色:气味:生物降解性:GHS危险性类别警示词:警告危险性说明:H315 - 造成皮肤刺激。
H319 - 造成严重眼刺激。
H335 - 可能造成呼吸道刺激。
H413 - 可能对水生生物造成长期持续有害影响。
:防范说明标签要素象形图皮肤腐蚀/刺激 - 类别 2严重眼损伤/眼刺激 - 类别 2AH335特异性靶器官毒性 一次接触 (呼吸道刺激) - 类别 3H413危害水生环境一长期危险 - 类别 4P273 - 避免释放到环境中。


熊去氧胆酸杂质谱
熊去氧胆酸是熊胆中的一种重要成分,具有多种药理活性。
为了对熊去氧胆酸进行分析,可以使用质谱技术。
质谱技术是一种化学分析方法,通过测量样品中的离子质量和丰度来确定化合物的分子结构和组成。
在熊去氧胆酸的分析中,可以使用质谱仪器将该化合物离子化,并通过对离子质量和丰度的测量来确定其分子结构。
质谱仪器通常包括两个主要部分:离子源和质谱仪。
离子源用于将样品中的分子转化为带电离子,常用的离子源包括电喷雾离子源(ESI)和化学电离源(CI)。
质谱仪则用于分离、激
发和检测离子。
在熊去氧胆酸的质谱分析中,通常会使用质谱仪中的质谱杂质分析模式,以确定待测样品中的杂质含量。
通过与已知标准品进行对比,可以定量分析熊去氧胆酸杂质的含量。
总的来说,熊去氧胆酸的质谱分析可以帮助确定其分子结构和组成,以及定量分析其杂质含量。
这种分析方法可以为熊胆制品的质量控制和药理研究提供重要的参考依据。

经上皮细胞消化后,ROCK 抑制剂Y27632可显著增加细胞粘附和增殖,提升hiPSCs-NECs 细胞存活率,抑制细胞凋亡过程[34]。
而且,CCK8细胞活力检测表明当Y27632的浓度达到5μmol/L 时,与浓度为8μmol/L 、10μmol/L 无明显差异。
同时,EdU 染色的结果也证明,当Y27632的浓度达到5μmol/L 时,细胞的增殖能力明显提高。
然而,iPSCs 体外分化过程中,Rho 激酶抑制剂浓度超过10μmol/L ,可能导致其分化为所有3个胚层(即外胚层、中胚层和内胚层),极大地损害了人iPSCs 定向诱导分化的能力[35]。
因此,在促进细胞贴壁存活后应及时去除Y27632,以免对后续的分化产生未知的影响。
本研究使用5μmol/L 浓度的Y27632促进hiPSCs-NECs 解离重新接种24h 后,更换新培养基,及时去除Y27632,持续诱导细胞至28d 后,可获得多巴胺能神经元前体细胞(DAPs ),高表达DAPs 标记物TH 、FOXA2、NURR1和Tuj1。
因此加入浓度为5μmol/L 的Y27632,未对后续iPSCs-DAPs 诱导产生明显的影响。
综上所述,本研究在人iPSCs 定向诱导DAPs 细胞过程中,在第1阶段人iPSC-NECs 解离重新接种时加入5μmol/L 的Y27632,可显著提高iPSCs-NECs 解离后的贴壁、存活和增殖,并且可减少凋亡细胞的比例,从而增加了后续分化过程中的细胞数量,间接的提高了分化效率。
24h 后去除Y27632,未对后续体外定向诱导iPSCs-DAPs 的分化潜能产生影响。
因此,在培养系统中补充ROCK 抑制剂Y27632提供了一种简单、高效的方法,可用于获得大量高纯度人iPSCs 衍生的多巴胺能神经前体细胞,这将为帕金森病的细胞移植治疗提供重要的细胞来源。
参考文献:[1]Wirdefeldt K,Adami HO,Cole P,et al.Epidemiology and etiology ofParkinson's disease:a review of the evidence [J ].Eur J Epidemiol,2011,26(Suppl 1):S1-S58.[2]Connolly BS,Lang AE.Pharmacological treatment of Parkinson图5人iPSCs-DAPs 的表面特征标记物的表达Fig.5Characteristics of hiPSCs-DAPs at stage 2.A :After treatment with different concentrations of Y27632(0,3,5,8and 10μmol/L),the morphological changes of hiPSCs-NECs were observed after further induction for 28days.B ,C :Immunofluorescence results show that hiPSCs-DAPs express the specific markers of midbrain dopamine neurons Nurr1(green),TH(Red),Tuj1(green)and FOXA2(Red)(×200).0μmol/L3μmol/L5μmol/L8μmol/L10μmol/LNurr1/FOXA2/DAPI/MergeTuj1/TH/DAPI/MergeBCA50μm50μm 50μm 50μm 50μm 50μm50μm100μm100μm 100μm 100μm 100μm J South Med Univ,2024,44(2):236-243··242disease:a review[J].JAMA,2014,311(16):1670-83.[3]Armstrong MJ,Okun MS.Diagnosis and treatment of parkinson disease:a review[J].JAMA,2020,323(6):548-60.[4]Zeuner KE,Schäffer E,Hopfner F,et al.Progress of pharmacological approaches in Parkinson's disease[J].Clin Pharmacol Ther,2019, 105(5):1106-20.[5]Parmar M.Towards stem cell based therapies for Parkinson's disease [J].Development,2018,145(1):dev156117.[6]Antonini A,Moro E,Godeiro C,et al.Medical and surgical management of advanced Parkinson's disease[J].Mov Disord,2018, 33(6):900-8.[7]Sonntag KC,Song B,Lee N,et al.Pluripotent stem cell-based therapy for Parkinson's disease:current status and future prospects [J].Prog Neurobiol,2018,168:1-20.[8]Xu PB,He H,Gao QQ,et al.Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcomes in a Parkinson's disease model[J].J Clin Invest,2022,132(14):e156768.[9]Fischbach GD,McKhann GM.Cell therapy for Parkinson's disease [J].N Engl J Med,2001,344(10):763-5.[10]Xiong M,Tao YZ,Gao QQ,et al.Human stem cell-derived neurons repair circuits and restore neural function[J].Cell Stem Cell,2021, 28(1):112-26.e6.[11]Song B,Cha Y,Ko S,et al.Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models[J].J Clin Invest,2020,130(2):904-20.[12]Kikuchi T,Morizane A,Doi D,et al.Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model[J].Nature,2017,548(7669):592-6.[13]Chen DD,Fu WY,Zhuang WX,et al.Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson's disease[J].J Neurosci Res,2017,95(3):907-17.[14]Kirkeby A,Parmar M,Barker RA.Strategies for bringing stem cell-derived dopamine neurons to the clinic:a European approach (STEM-PD)[J].Prog Brain Res,2017,230:165-90.[15]Schweitzer JS,Song B,Herrington TM,et al.Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease[J].N Engl J Med,2020,382(20):1926-32.[16]Chen GK,Hou ZG,Gulbranson DR,et al.Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells[J].Cell Stem Cell,2010,7(2):240-8.[17]Ohgushi M,Matsumura M,Eiraku M,et al.Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells[J].Cell Stem Cell,2010,7(2):225-39.[18]Wang XF,Lin G,Martins-Taylor K,et al.Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells[J].J Biol Chem, 2009,284(49):34054-64.[19]Watanabe K,Ueno M,Kamiya D,et al.A ROCK inhibitor permits survival of dissociated human embryonic stem cells[J].Nat Biotechnol,2007,25(6):681-6.[20]Kim MH,Takeuchi K,Kino-Oka M.Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture[J].JBiosci Bioeng,2019,127(3):372-80.[21]Gao LJ,Nath SC,Jiao XY,et al.Post-Passage rock inhibition induces cytoskeletal aberrations and apoptosis in Human embryonic stemcells[J].Stem Cell Res,2019,41:101641.[22]Koch JC,Tatenhorst L,Roser AE,et al.ROCK inhibition in models of neurodegeneration and its potential for clinical translation[J].Pharmacol Ther,2018,189:1-21.[23]Kamishibahara Y,Kawaguchi H,Shimizu N.Rho kinase inhibitor Y-27632promotes neuronal differentiation in mouse embryonic stemcells via phosphatidylinositol3-kinase[J].Neurosci Lett,2016,615:44-9.[24]Xue ZW,Shang XM,Xu H,et al.Rho-associated coiled kinase inhibitor Y-27632promotes neuronal-like differentiation of adulthuman adipose tissue-derived stem cells[J].Chin Med J,2012,125(18):3332-5.[25]Koyanagi M,Takahashi J,Arakawa Y,et al.Inhibition of the Rho/ ROCK pathway reduces apoptosis during transplantation ofembryonic stem cell-derived neural precursors[J].J Neurosci Res,2008,86(2):270-80.[26]Xu JJ,Li YY,Zhu H,et al.Therapeutic function of a novel rat induced pluripotent stem cell line in a6-OHDA-induced rat modelof Parkinson's disease[J].Int J Mol Med,2022,50(6):140.[27]Kawasaki H,Mizuseki K,Nishikawa S,et al.Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derivedinducing activity[J].Neuron,2000,28(1):31-40.[28]Yamanaka S.Pluripotent stem cell-based cell therapy-promise and challenges[J].Cell Stem Cell,2020,27(4):523-31.[29]Kriks S,Shim JW,Piao JH,et al.Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson'sdisease[J].Nature,2011,480(7378):547-51.[30]Yamamoto T,Ugawa Y,Kawamura M,et al.Modulation of micro-environment for controlling the fate of periodontal ligament cells:the role of Rho/ROCK signaling and cytoskeletal dynamics[J].JCell Commun Signal,2018,12(1):369-78.[31]Kurosawa H.Application of Rho-associated protein kinase(ROCK) inhibitor to human pluripotent stem cells[J].J Biosci Bioeng,2012,114(6):577-81.[32]Croze RH,Thi WJ,Clegg DO.ROCK inhibition promotes attac-hment,proliferation,and wound closure in human embryonic stemcell-derived retinal pigmented epithelium[J].Transl Vis SciTechnol,2016,5(6):7.[33]Wu YH,Shu JH,He CW,et al.ROCK inhibitor Y27632promotes proliferation and diminishes apoptosis of marmoset inducedpluripotent stem cells by suppressing expression and activity ofcaspase3[J].Theriogenology,2016,85(2):302-14.[34]Ishida M,Sugita S,Makabe K,et al.A ROCK inhibitor promotes graft survival during transplantation of iPS-cell-derived retinal cells [J].Int J Mol Sci,2021,22(6):3237.[35]Jiang B,Ou WQ,Shamul JG,et al.Rock inhibitor may compromise human induced pluripotent stem cells for cardiac differentiation in3D[J].Bioact Mater,2022,9:508-22.(编辑:吴锦雅) J South Med Univ,2024,44(2):236-243··243Total saponins of Panax japonicus alleviates CCl 4-induced acute liver injury in rats by regulating the PI3K/AktNF-κB signaling pathwayWU Guangyang 1,2,SONG Tianli 1,3,TANG Lang 1,WANG Yiming 1,LIU Xu 1,HUANG Sheng 1,31Department of Medicine,3Hubei Provincial Key Laboratory of Occurrence and Intervention of Rheumatic diseases,Hubei Minzu University,Enshi 445000,China;2Hubei Enshi College,Enshi 445000,China摘要:目的探讨土家族药用植物竹节参提取物总皂苷对CCl 4致急性肝损伤的保护作用及潜在的药理学机制。