盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察
- 格式:pdf
- 大小:184.90 KB
- 文档页数:2
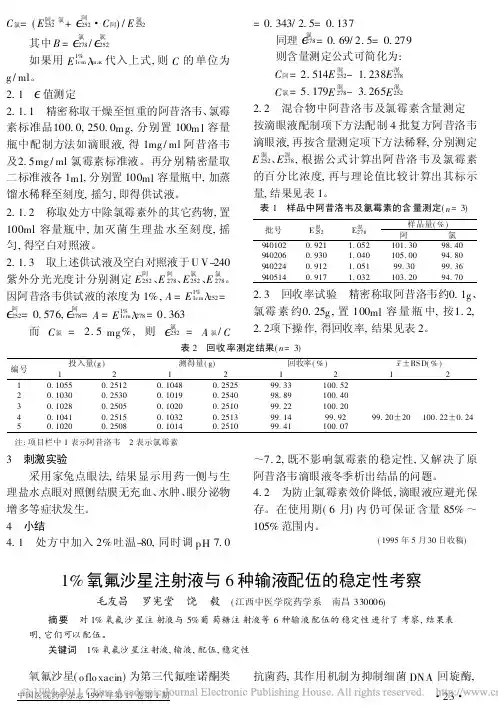
C 氯=(E 阿+氯252+ 阿252・C 阿)/E 氯252其中B = 氯278/ 氯252如果用E 1%1cm max 代入上式,则C 的单位为g /ml 。
2.1 值测定2.1.1 精密称取干燥至恒重的阿昔洛韦、氯霉素标准品100.0,250.0m g ,分别置100m l 容量瓶中配制方法如滴眼液,得1mg /ml 阿昔洛韦及2.5mg /ml 氯霉素标准液。
再分别精密量取二标准液各1m l ,分别置100ml 容量瓶中,加蒸馏水稀释至刻度,摇匀,即得供试液。
2.1.2 称取处方中除氯霉素外的其它药物,置100ml 容量瓶中,加灭菌生理盐水至刻度,摇匀,得空白对照液。
2.1.3 取上述供试液及空白对照液于U V-240紫外分光光度计分别测定E 阿252、E 阿278、E 氯252、E 氯278。
因阿昔洛韦供试液的浓度为1%,A =E 1%1cm 252=阿252=0.576, 阿278=A =E 1%1cm 278=0.363而C 氯= 2.5mg %,则 氯252=A 氯/C=0.343/2.5=0.137同理氯278=0.69/2.5=0.279则含量测定公式可简化为:C 阿=2.514E 混252-1.238E 混278C 氯=5.179E 混278-3.265E 混2522.2 混合物中阿昔洛韦及氯霉素含量测定 按滴眼液配制项下方法配制4批复方阿昔洛韦滴眼液,再按含量测定项下方法稀释,分别测定E 混252、E 混278,根据公式计算出阿昔洛韦及氯霉素的百分比浓度,再与理论值比较计算出其标示量,结果见表1。
表1 样品中阿昔洛韦及氯霉素的含量测定(n =3)批号E 混252E 混278样品量(%)阿氯9401020.921 1.052101.3098.409402060.930 1.040105.0094.809402240.912 1.05199.3099.369405140.9171.032103.2094.702.3 回收率试验 精密称取阿昔洛韦约0.1g 、氯霉素约0.25g ,置100ml 容量瓶中,按1.2,2.2项下操作,得回收率,结果见表2。


甲磺酸培氟沙星注射液与利巴韦林注射液的配伍稳定性倪海镜;戴维群;徐丽君
【期刊名称】《吉林大学学报(医学版)》
【年(卷),期】2003(029)002
【摘要】目的:观察甲磺酸培氟沙星注射液与利巴韦林注射液的配伍稳定性.方法:采用紫外分光光度法,定时定量取样,在各自的λmax处测定其A值,计算百分含量,判断两者配伍后的稳定性.结果:将甲磺酸培氟沙星注射液及利巴韦林注射液同时加入生理盐水、复方氯化钠、5%及10%葡萄糖、5%葡萄糖氯化钠等输液中,在一定时间范围内是稳定的.结论:甲磺酸培氟沙星与利巴韦林注射液在本实验条件下可以配伍应用于上述各输液中.
【总页数】2页(P220-221)
【作者】倪海镜;戴维群;徐丽君
【作者单位】吉林大学中日联谊医院药剂科,吉林,长春,130031;吉林大学中日联谊医院药剂科,吉林,长春,130031;吉林省人民医院药剂科
【正文语种】中文
【中图分类】R942
【相关文献】
1.利巴韦林注射液与克林霉素磷酸酯氯化钠注射液配伍的稳定性考察 [J], 朱雅艳;应小飞;田伟强;朱虹云
2.利巴韦林注射液与盐酸雷尼替丁注射液配伍的稳定性考察 [J], 温坚;夏敬民
3.盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察 [J], 李红月;罗朝利;宋畅
4.利巴韦林注射液与其他4种注射液配伍稳定性研究 [J], 侯巍;滕杨
5.甲磺酸帕珠沙星与利巴韦林注射液在葡萄糖注射液中的配伍稳定性 [J], 姜芳宁;任洁
因版权原因,仅展示原文概要,查看原文内容请购买。

双波长分光光度法考察利巴韦林与维生素C配伍的稳定性
伦新强
【期刊名称】《数理医药学杂志》
【年(卷),期】2003(016)004
【摘要】为考察利巴韦林与维生素C注射液在四种常用输液中配伍的稳定性,在室温25℃条件下,将利巴韦林与维生素C按临床用药浓度在5%葡萄糖、10%葡萄糖、葡萄糖氯化钠、0.9%氯化钠注射液中配伍后,观察外观变化及测定pH值,同时采用双波长分光光度法测定两药含量.结果显示,两药配伍液在0~8h内其外观、pH值
及含量均无明显变化.
【总页数】2页(P338-339)
【作者】伦新强
【作者单位】广西柳州地区人民医院,柳州545002
【正文语种】中文
【中图分类】R912
【相关文献】
1.双波长分光光度法考察注射用加替沙星与头孢吡肟配伍的稳定性 [J], 陈文钦;徐璐敏;华俊彦;冯炎林
2.双波长分光光度法考察二羟丙茶碱注射液与盐酸莫西沙星氯化钠注射液配伍的稳定性 [J], 张丽梅;李俊;武俊
3.盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察 [J], 李红月;罗朝利;宋畅
4.双波长分光光度法考察先福吡兰和甲硝唑注射液配伍的稳定性 [J], 林祖文;罗宇
芬
5.阿奇霉素氯化钠注射液与利巴韦林、维生素C配伍的稳定性 [J], 朱春来;弈国华因版权原因,仅展示原文概要,查看原文内容请购买。

长治市三宝生化药业有限公司编号SBB2.8.5.6利巴韦林注射液生产工艺验证方案长治市三宝生化药业有限公司方案制订签名日期方案会签签名日期生产技术部签名日期验证小组签名日期方案批准质量保证部日期目录1.概述`````````````````````````````````````````````````````````````````````````````````````````````````````````````````````````````4` 1.1.产品简述``````````````````````````````````````````````````````````````````````````````````````````````````````````````4 1.2.处方及依据``````````````````````````````````````````````````````````````````````````````````````````````````````````41.3.生产工艺流``````````````````````````````````````````````````````````````````````````````````````````````````````````5`2.验证目的````````````````````````````````````````````````````````````````````````````````````````````````````````````````````53.验证的范围```````````````````````````````````````````````````````````````````````````````````````````````````````````````64.验证各部门职责及组织结构```````````````````````````````````````````````````````````````````````````````65.验证准备````````````````````````````````````````````````````````````````````````````````````````````````````````````````````76.验证内容及实施``````````````````````````````````````````````````````````````````````````````````````````````````````8` 6.1.洗瓶工序````````````````````````````````````````````````````````````````````````````````````````````````````````````8 6.2.配制工序```````````````````````````````````````````````````````````````````````````````````````````````````````````12 6.3.灌封工序```````````````````````````````````````````````````````````````````````````````````````````````````````````15 6.4.灭菌工序```````````````````````````````````````````````````````````````````````````````````````````````````````````20 6.5.灯检工序```````````````````````````````````````````````````````````````````````````````````````````````````````````24 6.6.包装工序```````````````````````````````````````````````````````````````````````````````````````````````````````````266.7.成品检验结果``````````````````````````````````````````````````````````````````````````````````````````````````287.偏差分析``````````````````````````````````````````````````````````````````````````````````````````````````````````````````298.验证结论``````````````````````````````````````````````````````````````````````````````````````````````````````````````````299.附表````````````````````````````````````````````````````````````````````````````````````````````````````````````````````````````29 9.1. 设备一览表及生产能力```````````````````````````````````````````````````````````````````````````````30 9.2.设备性能验证确认及检查情况表```````````````````````````````````````````````````````````````31 9.3参加验证人员培训情况检查表````````````````````````````````````````````````````````````````````32 9.4.厂房与公用设施验证的确认和检查情况表`````````````````````````````````````````````34 9.5.空气净化系统、工艺用水系统验证的确认和检查情况表`````````````````35 9.6.计量器具检查情况表```````````````````````````````````````````````````````````````````````````````````````36 9.7.三批(按四批准备)验证使用的原料、辅料和安瓿供应商确认及检查情况表`````````````````````````````````````````````````````````````````````````````37 9.8.质量检验系统验证和准备情况表```````````````````````````````````````````````````````````````38 9.9.检验仪器检查情况表``````````````````````````````````````````````````````````````````````````````````````39 9.10检验试剂检查情况表````````````````````````````````````````````````````````````````````````````````````40 9.11质量监控点、监控内容、监控方法、监控频次表`````````````````````````````411.概述1.1.利巴韦林注射液(1ml:100mg)常温状态下是无色的澄明液体,属抗病毒药,用于呼吸道合胞病毒引起的病毒性肺炎与支气管炎。


洛美沙星注射液与利巴韦林注射液配伍的稳定性
王江红
【期刊名称】《徐州医学院学报》
【年(卷),期】2001(021)001
【摘要】目的研究洛美沙星注射液与利巴韦林注射液配伍的稳定性.方法在25、37℃下观察配伍液的外观、pH值、紫外光谱的变化,用紫外分光光度法测定洛美沙星、利巴韦林的含量.结果各配伍液的外观、pH值及紫外图谱及含量均无显著变化.结论洛美沙星注射液可与利巴韦林注射液配伍使用.
【总页数】2页(P79-80)
【作者】王江红
【作者单位】徐州医学院附属医院药剂科,
【正文语种】中文
【中图分类】R942
【相关文献】
1.利巴韦林注射液与克林霉素磷酸酯氯化钠注射液配伍的稳定性考察 [J], 朱雅艳;应小飞;田伟强;朱虹云
2.盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察 [J], 李红月;罗朝利;宋畅
3.利巴韦林注射液与其他4种注射液配伍稳定性研究 [J], 侯巍;滕杨
4.天门冬氨酸洛美沙星与利巴韦林注射液配伍的稳定性 [J], 陈漪
5.甲磺酸帕珠沙星与利巴韦林注射液在葡萄糖注射液中的配伍稳定性 [J], 姜芳宁;任洁
因版权原因,仅展示原文概要,查看原文内容请购买。

利巴韦林注射液配伍禁忌是什么呢医学上很多的药物都是有配伍禁忌的,医护人员再给病人开药物的时候都是需要掌握一下药性,不同的药物药性也是不同的,每一种药物并不是单一的,而且疾病需要靠多种药物的综合作用才能够有效果,那么利巴韦林注射液配伍禁忌有哪些呢,作为医护人员关于这个配伍禁忌问题下面详细的介绍一下。
有些药品配伍使药物的治疗作用减弱,导致治疗失败;有些药品配伍使副作用或毒性增强,引起严重不良反应;还有些药品配伍使治疗作用过度增强,超出了机体所能耐受的能力,也可引起不良反应,乃至危害病人等。
那么,你是否知道? 利巴韦林注射液属于广谱抗病毒药。
体外具抑制呼吸道合胞病毒、流感病毒、甲肝病毒、腺病毒等多种病毒生长的作用,其机制不全清楚。
利巴韦林注射液并不改变病毒吸附、侵入和脱壳,也不诱导干扰素的产生。
药物进入被病毒感染的细胞后迅速磷酸化,其产物作为病毒合成酶的竞争性抑制剂,抑制肌苷单磷酸脱氢酶、流感病毒RNA聚合酶和mRNA鸟苷转移酶,从而引起细胞内鸟苷三磷酸的减少,损害病毒RNA和蛋白合成,使病毒的复制与传播受抑。
对呼吸道合胞病毒也可能具免疫作用及中和抗体作用。
利巴韦林注射液主要用于呼吸道合胞病毒(RSV)引起的病毒性肺炎与支气管炎。
流行性出血热和拉沙热的预防和治疗,发热早期应用利巴韦林注射液能缩短发热期,减轻肾脏与血管损害及中毒症状。
局部应用可治疗单纯疱疹病毒性角膜炎。
利巴韦林注射液与齐多夫定同用时有拮抗作用,因利巴韦林注射液可抑制齐多夫定转变成活性型的磷酸齐多夫定。
所以,是不建议利巴韦林注射液与齐多夫定同时使用的。
上述内容仅供大家参考,如果您还想了解到更多的,可以登录康爱多药店进行咨询的。
康爱多通过建立药嘱、药物咨询、疾病用药问询等服务,与你一起关注健康,多一分关爱,多一些健康。
下面,笔者为大家推荐另外两种具有同样疗效的药品,供大家参考:乙酰半胱氨酸泡腾片:用于治疗分泌大量浓稠痰液的慢性阻塞性肺病(COPD)、慢性支气管炎(CB)、肺气肿(PE)等慢性呼吸系统感染。


盐酸洛美沙星氯化钠注射液与甲硝唑注射液配伍的稳定性实验秦安龙;李蓉;于红艳
【期刊名称】《黑龙江医药》
【年(卷),期】2008(021)006
【摘要】目的:考察盐酸洛美沙星氯化钠注射液与甲硝唑注射液的配伍稳定性.方法:用高效液相色谱法测定25℃、37℃条件下放置6小时内配伍液中两组分含量,并观察输液的外观及pH值的变化.结果:在25℃、37℃下,盐酸洛美沙星氯化钠注射液与甲硝唑注射液配伍稳定.结论:可以配伍使用.
【总页数】2页(P23-24)
【作者】秦安龙;李蓉;于红艳
【作者单位】江苏省苏北人民医院;江苏省苏北人民医院;扬州大学医学院,扬州市,225002
【正文语种】中文
【中图分类】R969.2
【相关文献】
1.盐酸洛美沙星注射液与5种中药注射液配伍稳定性实验 [J], 汪细和;黄帮华;王世平
2.头孢噻肟钠与甲硝唑注射液的配伍稳定性实验 [J], 肖华;汤韧
3.头孢噻肟钠与甲硝唑注射液的配伍稳定性实验 [J], 李勇文
4.盐酸洛美沙星注射液与注射用头孢拉定配伍的稳定性实验 [J], 何志义;吴燕萍;等
5.环丙沙星注射液与甲硝唑注射液配伍的稳定性实验 [J], 宋祺;王志朝;马莉;杜蓉;符旭东
因版权原因,仅展示原文概要,查看原文内容请购买。
利巴韦林注射液与48种药物的配伍稳定性研究发表时间:2016-12-23T14:18:00.503Z 来源:《航空军医》2016年第23期作者:吴玉明[导读] 对利巴韦林注射液与48种药物的配伍稳定性进行分析研究,为临床用药的合理性提供了支持。
冷水江市中医医院湖南冷水江 417500【摘要】目的:对利巴韦林注射液与48种药物的配伍稳定性进行分析研究,为临床用药的合理性提供了支持。
方法:使用万方数据库、知网以及维普,输入利巴韦林注射液、稳定以及配伍这些关键词,对2000年至今与利巴韦林注射液和其他各种物配伍稳定性有关的文献进行检索,并对其进行分析。
结果:氨苄西林钠舒巴坦钠和利巴韦林进行配伍后,随着时间的延长颜色会从无色变为淡黄色、含量变化、pH值变化明显。
多烯磷脂酰胆碱与其配伍后会迅速发生反应,产生橙黄色沉淀,利巴韦林注射液与其他药物配伍后pH值、外观、含量等变化不大。
结论:氨苄西林钠舒巴坦钠和利巴韦林配伍后不稳定;多烯磷脂酰胆碱和利巴韦林配伍不合适;在一定时间内,余下46种药物和利巴韦林可配伍。
【关键词】利巴韦林注射液;稳定性;配伍[Abstract] objective:to 48 species of ribavirin injection and drug compatibility of stability analysis,provide the support for the rationality of clinical medication. Methods:use of a database of ten thousand,hownet and VIP,input and ribavirin injection,stability and compatibility of these keywords,the since 2000 with ribavirin injection and other various compatibility stability on literature retrieval,and carries on the analysis. Results:shu ba jotham sodium ampicillin sodium and ribavirin compatibility,over time the color will change from colorless to pale yellow,content,pH value change is obvious. Polyene phosphatidyl choline and its compatibility to react quickly and produce orange precipitation,ribavirin injection after compatibility with other drugs,pH,appearance,content,etc. Conclusion:shu ba jotham ampicillin sodium sodium and ribavirin compatibility after unstable;Polyene phosphatidyl choline and ribavirin compatibility is not appropriate;In a certain period of time,the remaining 46 kinds of drugs and compatibility and wei forests. [Key words] ribavirin injection;Stability;compatibility利巴韦林是一种合成的核苷类广谱抗病毒药物,对治疗由呼吸道合胞病毒引起的病毒性肺炎及支气管炎疗效明显,对呼吸道合胞病毒可以起到免疫及中和抗体的益处。
利巴韦林注射液与青霉素钠或头孢唑林钠配伍的稳定性
李春艳
【期刊名称】《中华医学写作杂志》
【年(卷),期】2004(011)014
【摘要】利巴韦林(三氮唑核苷)注射液是一种广谱抗病毒药物,对多种RNA病毒及DNA病毒有抑制作用。
病毒性疾病如重症乙型脑炎、重症肝炎及呼吸道、流感、麻疹的病毒性感染伴有继发性细菌感染时,为预防或治疗细菌性感染,临床上往往将Rib注射液与注射用青霉素钠(PN)或头孢林钠(CN)配合应用,而配伍后各药稳
定性如何,未见报道。
我们为配合临床治疗,对Rib注射液与PN或CN配伍的稳定性,进行了实验研究,现报告如下。
【总页数】2页(P1252-1253)
【作者】李春艳
【作者单位】黑龙江省逊克县人民医院164400
【正文语种】中文
【中图分类】R969
【相关文献】
1.注射用头孢唑林钠与利巴韦林注射液在输液中配伍的稳定性考察 [J], 韦仕勇
2.注射用头孢唑林钠溶液稳定性及配伍稳定性研究 [J], 赖可;陶静;邓盛齐;张彧
3.青霉素钠和头孢唑林钠与脂肪乳配伍时的含量测定 [J], 李颖华;马玉樊;王荧;张
志刚;张宏;卢婷利;陈涛
4.盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察 [J], 李红月;罗朝利;宋畅
5.注射用甲泼尼龙琥珀酸钠与利巴韦林注射液配伍稳定性因素的研究 [J], 何心;陈晓慧;李桂丹
因版权原因,仅展示原文概要,查看原文内容请购买。
天门冬氨酸洛美沙星与更昔洛韦注射液配伍稳定性考察
陈漪
【期刊名称】《实用药物与临床》
【年(卷),期】2004(7)4
【摘要】目的在5℃,20℃,37℃下考察天门冬氨酸洛美沙星与更昔洛韦注射在5%葡萄糖及0.9%氯化钠中的配伍稳定性.方法采用双波长-等吸收点光度法测定配伍液中各自含量的变化情况.结果配伍液在上述各温度下,0~6 h内其外观、浊度、pH值、含量及吸收光谱图均无明显变化.结论上述两种注射液在6 h内稳定,可配伍使用.
【总页数】2页(P77-78)
【作者】陈漪
【作者单位】宁波妇女儿童医院药剂科,浙江,宁波,315012
【正文语种】中文
【中图分类】R927
【相关文献】
1.盐酸洛美沙星与甲硝唑氯化钠注射液配伍稳定性考察 [J], 杨青青
2.盐酸洛美沙星注射液与利巴韦林注射液配伍稳定性考察 [J], 李红月;罗朝利;宋畅
3.洛美沙星注射液与四种止血药配伍稳定性考察 [J], 卞宏芳;朱华
4.洛美沙星注射液与四种止血药配伍稳定性考察 [J], 卞宏芳;朱华
5.天门冬氨酸洛美沙星与利巴韦林注射液配伍的稳定性 [J], 陈漪
因版权原因,仅展示原文概要,查看原文内容请购买。
洛美沙星与24种药物配伍的稳定性洛美沙星(Lomefloxacin)是第三代喹诺酮类广谱抗生素,是主要作用于革兰阴性菌的抗菌药物,对葡萄球菌等革兰阳性菌也有抗菌作用。
临床上用于治疗呼吸道、泌尿道、消化道、皮肤和软组织等感染。
洛美沙星在临床上应用日益广泛,与其他药物配伍也日渐增多,现就临床上常用药物与洛美沙星注射液的配伍进行简述,为临床合理用药提供参考。
1 洛美沙星注射液与其他药物配伍的变化(见表1)2 讨论洛美沙星注射液分别与0.9%氯化钠、葡萄糖氯化钠、5%葡萄糖、10%葡萄糖、20%甘露醇、乳酸钠林格、酚磺乙胺、维生素C、奥硝唑氯化钠、头孢拉定、更昔洛韦、替硝唑葡萄糖、利巴韦林、硫酸庆大霉素、硫酸妥布霉素等15种注射液配伍,在上述条件下,外观无颜色变化和沉淀、混浊现象,pH值及吸收光谱均未有明显改变,相对百分含量均在90.0%以上,说明这14种药物与加替沙星注射液配伍比较稳定,在临床上可以配伍给药,但配伍后的药液应尽快使用。
洛美沙星注射液与注射用阿昔洛韦配伍6h后阿昔洛韦相对百分含量<90%,二者不宜配伍。
洛美沙星注射液分别与氯唑西林钠、头孢哌酮钠、硫酸阿米卡星、磺胺嘧啶钠、地西泮、呋噻米、肝素钠及氢化可的松等注射剂按1:1等量置多孔点滴板混合后,生成白色沉淀,说明洛美沙星注射液与上述8种注射液不能配伍使用。
对与洛美沙星注射液不宜或不能配伍使用的药物,如临床需要,可在二药之间插输少量其他液体,以冲净原输液管中剩余药物或改输其他组的药液,来避免二者潜在的或直接的配伍反应。
另外,应多加注意,临床配伍时严禁用1支注射器反复抽取多种药物随意配伍。
参考文献:[1] 罗艳,肖琳. 盐酸洛美沙星与4种常用输液配伍的稳定性考察[J].衡阳医学院学报,2000,28(2):171.[2] 黄楚权,谢翠刁. 盐酸洛美沙星注射剂在不同输液中的稳定性[J].广东药学,2001,11(5):36.[3] 郭英芳,肖克来提. 盐酸洛美沙星注射液与2种药物配伍的稳定性[J].中国医院药学杂志,2000,20(1):57.[4] 朱玲仙,许凤娟. 注射用盐酸洛美沙星与奥硝唑氯化钠注射液配伍稳定性研究[J].实用药物与临床,2006,9(4):211.[5] 何志义,吴燕萍,陈灵. 盐酸洛美沙星注射液与注射用头孢拉定配伍的稳定性实验[J].遵义医学院学报,2000,23(4):365.[6] 陈漪. 天门冬氨酸洛美沙星与更昔洛韦注射液配伍稳定性考察[J].实用药物与临床,2004,7(4):77.[7] 陈灵,郑传痴,何志义,等.替硝唑葡萄糖注射液与洛美沙星注射液配伍的稳定性实验[J]. 遵义医学院学报,2000,23(3):284.[8] 陈春玲,朱华,贾玲昌,等. 洛美沙星注射液与注射用阿昔洛韦配伍稳定性考察[J].实用药物与临床,2006,9(3):134.[9] 王江红.洛美沙星注射液与利巴韦林注射液配伍的稳定性[J]. 徐州医学院学报,2001,21(1):79.[10] 张鹏飞,朱华. 洛美沙星注射液与3种氨基糖苷类药物配伍的稳定性[J]. 现代医药卫生,2006,22(24): 3756.。