Fulvestrant_COA_22211_MedChemExpress
- 格式:pdf
- 大小:92.27 KB
- 文档页数:1


WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。

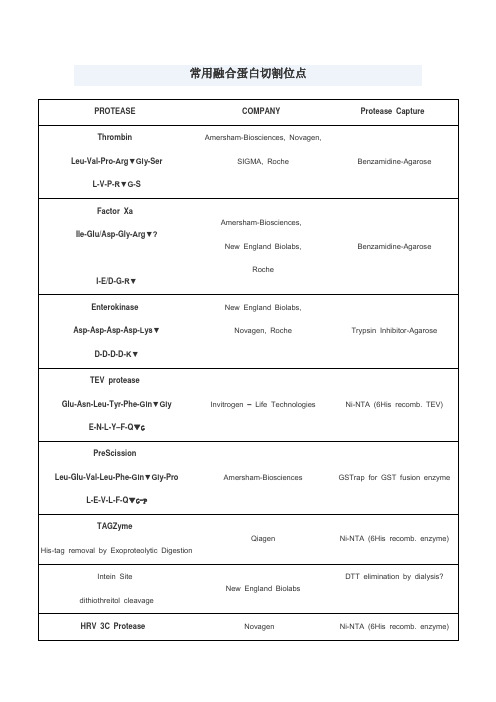

- 105 -*基金项目:广州市卫生健康科技项目(20211A010005)①广州市第一人民医院 广东 广州 510180通信作者:陈国东参芪扶正注射液联合经动脉化疗栓塞治疗中晚期肝癌患者的效果及安全性*李西山① 陈国东①【摘要】 目的:分析参芪扶正注射液联合经动脉化疗栓塞(TACE)治疗中晚期肝癌患者的效果和安全性。
方法:选择2021年1月—2022年12月于广州市第一人民医院接受治疗的98例中晚期肝癌患者为研究对象,采用随机数表法均分为研究组和对照组,各49例。
对照组仅予以TACE 治疗,研究组在TACE 的基础上予以静脉参芪扶正注射液联合治疗。
比较两组的临床有效率、肝功能指标[谷丙转氨酶(ALT)、谷草转氨酶(AST)]、免疫学指标(CD3+、CD4+、CD8+和CD4+/CD8+)、不良反应发生率和生活质量。
结果:研究组临床总有效率为79.59%,高于对照组的63.27%,差异有统计学意义(P <0.05)。
术后2周,两组ALT、AST 均低于术前1 d,且研究组上述指标均低于对照组,差异有统计学意义(P <0.05)。
术后2周,研究组CD3+、CD4+和CD4+/CD8+高于术前1 d 和对照组,而CD8+水平低于术前1 d 和对照组,差异有统计学意义(P <0.05)。
研究组不良反应总发生率为30.61%,低于对照组的57.14%,差异有统计学意义(P <0.05)。
研究组生活质量改善率为91.84%,高于对照组的65.31%,差异有统计学意义(P <0.05)。
结论:参芪扶正注射液联合TACE 治疗能够提高中晚期肝癌患者的临床有效率,保护患者的肝功能,减少患者TACE 后不良反应,提高患者的免疫学水平和临床生活质量。
【关键词】 肝癌 参芪扶正注射液 经动脉化疗栓塞 疗效 不良反应 doi:10.14033/ki.cfmr.2024.02.027 文献标识码 B 文章编号 1674-6805(2024)02-0105-04 Efficacy and Safety of Shenqi Fuzheng Injection Combined with Transarterial Chemoembolization in the Treatment of Patients with Middle and Advanced Liver Cancer/LI Xishan, CHEN Guodong. //Chinese and Foreign Medical Research, 2024, 22(2): 105-108 [Abstract] Objective: To analyze the efficacy and safety of Shenqi Fuzheng Injection combined with transarterial chemoembolization (TACE) in the treatment of patients with middle and advanced liver cancer. Method: A total of 98 patients with middle and advanced liver cancer who received treatment in Guangzhou First People's Hospital from January 2021 to December 2022 were selected as the study objects, and they were divided into study group and control group by random number table method, with 49 cases in each group. The control group was treated with TACE only, while the study group was treated with intravenous Shenqi Fuzheng Injection on the basis of TACE. The clinical effective rate, liver function indexes [alanine aminotransferase (ALT), aspartate aminotransferase (AST)], immunological indexes (CD3+, CD4+, CD8+ and CD4+/CD8+), incidence of adverse reactions and quality of life of the two groups were compared. Result: The total clinical effective rate of the study group was 79.59%, which was higher than 63.27% of the control group, the difference was statistically significant (P <0.05). At 2 weeks after surgery, ALT and AST in both groups were lower than those 1 d before surgery, and the above indexes in the study group were lower than those in the control group, the differences were statistically significant (P <0.05). At 2 weeks after surgery, the levels of CD3+, CD4+ and CD4+/CD8+ in the study group were higher than those 1 d before surgery and control group, while CD8+ levels were lower than those 1 d before treatment and control group, the differences were statistically significant (P <0.05). The total incidence of adverse reactions in the study group was 30.61%, which was lower than 57.14% in the control group, and the difference was statistically significant (P <0.05). The improvement rate of quality of life in the study group was 91.84%, which was higher than 65.31% in the control group, and the difference was statistically significant (P <0.05). Conclusion: Shenqi Fuzheng Injection combined with TACE can improve the clinical effective rate of patients with middle and advanced liver cancer, protect the liver function of patients, reduce the adverse reactions after TACE, and improve the immunological level and clinical quality of life. [Key words] Liver cancer Shenqi Fuzheng Injection Transarterial chemoembolization Efficacy Adverse reactions First-author's address: Guangzhou First People's Hospital, Guangzhou 510180, China 肝癌是世界上癌症相关死亡的最常见原因之一,我国超过80%的肝癌患者在确诊时已为中晚期[1]。

咖啡酸苯乙酸酯对丙烯醛雾化诱导的小鼠早期气道重塑的干预作用李艳萍;刘代顺;文富强;冯玉麟;李万成;刘维佳【期刊名称】《华西医学》【年(卷),期】2007(22)2【摘要】目的丙烯醛雾化吸入2周诱导小鼠气道重塑模型,研究NF-κB易位抑制剂咖啡酸苯乙酸酯(CAPE)对小鼠早期气道重塑的干预作用。
方法24只C57BL/6小鼠随机分为4组(每组6只)正常对照组、单纯CAPE组(腹腔内注射CAPE30mg/kg,隔日一次)、丙烯醛组(丙烯醛雾化吸入2h×3次/d,共2周)、丙烯醛雾化合并CAPE干预组(雾化吸入丙烯醛同时腹腔内注射CAPE)。
于实验第2周采取各组小鼠肺组织和支气管肺泡灌洗液(BALF)。
肺组织HE、MASSON染色和羟脯氨酸定量观察气道平滑肌增生、气道上皮下及管周肺组织胶原沉积情况,Ashcroft评分计算HE染色下小鼠气道及管周肺组织的纤维化严重程度;BALF 行细胞计数观察气道炎症反应,ELISA测定BALF中TGF-β1含量。
结果(1)丙烯醛组气道炎症反应,平滑肌增生及气道上皮下胶原沉积较正常对照组显著增高(P<0.05)。
(2)丙烯醛雾化合并CAPE干预组气道炎症反应及管周肺组织平滑肌增生,上皮下基底膜沉积较丙烯醛组明显减轻(P<0.05)。
结论NF-κB抑制剂CAPE能够减少致纤维化因子TGF-β1的释放,减轻早期气道炎症反应及重塑的进程。
【总页数】3页(P321-323)【关键词】咖啡酸苯乙酸酯;气道重塑;丙烯醛;NF—κB抑制剂【作者】李艳萍;刘代顺;文富强;冯玉麟;李万成;刘维佳【作者单位】四川大学华西医院呼吸内科【正文语种】中文【中图分类】R562【相关文献】1.桔梗皂苷对慢性支气管炎小鼠气道重塑的干预作用研究 [J], 陈勤;朱敏;李杨;谢华;张尧生2.TSLP-OX40信号通路对哮喘小鼠气道重塑的影响及地塞米松的干预作用 [J], 宫静;吴根英;高修霞3.布地奈德干预对哮喘小鼠NF-κB/TGF-β1通路及早期气道重塑的影响 [J], 邓鹏辉;马常亭;张健;高明霞;李鸿佳;张才擎4."补肺益肾、养血祛瘀"法干预支气管哮喘小鼠气道炎症及气道重塑的作用机制[J], 王盛隆;刘海鑫;董爱爱;白丽;陈慧婷;刘灵;张亚妮;范嘉琦5.依拉普利对丙烯醛诱导的气道炎症干预作用的研究 [J], 徐治波;冯玉麟;肖军;唐永江;秋婷因版权原因,仅展示原文概要,查看原文内容请购买。

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-04-2017Print Date:Jul.-04-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :FebuxostatCatalog No. :HY-14268CAS No. :144060-53-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:TEI 6720; TMX 67Formula:C16H16N2O3SMolecular Weight:316.37CAS No. :144060-53-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。

·专家论坛·合成生物学在医药领域的应用进展李瀚纯王鑫瞿旭东王舒[弈柯莱生物科技(集团)股份有限公司上海 200241]摘要近20年来,合成生物学在生物回路构建、生物元件标准化,以及各种基因组/代谢工程工具和方法的开发方面不断取得突破。
合成生物学的快速发展正在改变生物技术行业的产业布局。
目前,合成生物技术已广泛应用于天然产物合成、医学、能源、工业等多个领域。
医药的需求也推动了合成生物学的发展,包括将体外催化技术应用于手性医药化学品的绿色制造,将异源途径整合到细胞中以有效生产药物等。
合成生物学凭藉更经济、环境友好等突出特点,将颠覆一部分传统医药的制造方式。
本文概要介绍合成生物学在手性医药化学品的绿色制造和植物天然产物的生物制造方面的应用进展。
关键词合成生物学 医药化学品 天然产物 萜类化合物 芳香族化合物中图分类号:Q819; O629.71 文献标志码:A 文章编号:1006-1533(2024)07-0024-08引用本文李瀚纯, 王鑫, 瞿旭东, 等. 合成生物学在医药领域的应用进展[J]. 上海医药, 2024, 45(7): 24-31; 55.Applications of synthetic biology in the pharmaceutical fieldLI Hanchun, WANG Xin, QU Xudong, WANG Shu(Abiochem Biotechnology Co., Ltd., Shanghai 200241, China)ABSTRACT In the past two decades, synthetic biology has made breakthroughs in the construction of biocircuits, the standardization of biological elements and the development of various genomic/metabolic engineering tools and approaches.Its rapid development is changing the industrial layout of biotechnology industry. At present, synthetic biotechnology has been widely used in many fields such as natural product synthesis, medicine, energy and industry. Pharmaceutical demands have also driven its development, including the application of in vitro catalytic technology in the green manufacturing of chiral pharmaceutical chemicals and the integration of heterologous pathways into designer cells to efficiently produce medicines and so on. Synthetic biology, with its more economical and environmentally friendly features, will subvert some traditional pharmaceutical manufacturing methods. This article reviews the applications of synthetic biology in the green manufacturing of chiral pharmaceutical chemicals and the biological manufacturing of natural plant products.KEY WORDS synthetic biology; pharmaceutical chemicals; natural products; terpenoids; aromatic compounds合成生物学是采用工程科学研究理念,对生物体进行定向设计、理性改造甚至创造新型生物体的一门学科。

DOI:10.16658/ki.1672-4062.2023.19.084德谷门冬双胰岛素注射液治疗2型糖尿病的疗效及安全性研究戴卉,张开凤,朱凤丽江苏省镇江市丹徒区人民医院内分泌科,江苏镇江212000[摘要]目的探讨德谷门冬双胰岛素注射液在2型糖尿病中的效果以及安全性。
方法选取2022年1月—2023年7月江苏省镇江市丹徒区人民医院收治的62例2型糖尿病患者为研究对象,按随机数表法分为对照组(n=31)和观察组(n=31)。
对照组患者接受门冬胰岛素30注射液治疗,观察组患者接受德谷门冬双胰岛素注射治疗。
对比两组患者临床疗效、血糖变化和不良反应发生率。
结果观察组治疗有效为96.77%,高于对照组的77.42%,差异有统计学意义(χ2=5.167,P=0.023)。
治疗前,两组患者血糖水平比较,差异无统计学意义(P>0.05);治疗后,两组患者血糖水平均改善,且观察组血糖指标低于对照组,差异有统计学意义(P< 0.05)。
观察组不良反应发生率低与对照组,差异有统计学意义(P<0.05)。
结论德谷门冬双胰岛素的应用可以明显改善2型糖尿病患者血糖水平,疗效更为确切,且安全性更高,不会增加用药后不良反应。
[关键词] 2型糖尿病;德谷门冬双胰岛素;门冬胰岛素30注射液;安全性[中图分类号] R587 [文献标识码] A [文章编号] 1672-4062(2023)10(a)-0084-04Study on the Efficacy and Safety of Insulin Degludec and Insulin Aspart Injection in the Treatment of Type 2 Diabetes MellitusDAI Hui, ZHANG Kaifeng, ZHU FengliDepartment of Endocrinology, Zhenjiang Dantu District People's Hospital, Zhenjiang, Jiangsu Province, 212000 China [Abstract] Objective To explore the effect and safety of insulin degludec and insulin aspart injection in type 2 diabe⁃tes mellitus.Methods 62 patients of type 2 diabetes mellitus patients admitted to Zhenjiang Dantu District People's Hospital, Jiangsu Province from January 2022 to July 2023 were selected as study objects and divided into the control group (n=31) and the observation group (n=31) by taking the random number table method. The patients in the control group were treated with insulin aspart 30 injection and the patients in the observation group were treated with insulin degludec and insulin aspart injection. Compared the clinical efficacy, the changes in blood glucose and the incidence of adverse reactions between the two groups of patients.Results The treatment effectiveness of the observation group was 96.77%, which was higher than that of the control group, which was 77.42%, and the difference was statistically significant (χ2=5.167, P=0.023). There was no statistically significant difference in blood glucose levels between the two groups before treatment (P>0.05). After treatment, blood glucose levels improved in both groups, and the level of blood glucose in the observation group were lower than those in the control group, and the difference was statistically significant (P<0.05). The incidence of adverse reactions in the observation group was lower than that in the control group, and the difference was statistically significant (P<0.05).Conclusion The application of insulin degludec and in⁃sulin aspart can significantly improve the blood glucose level of patients with type 2 diabetes mellitus, the efficacy is more accurate, and the safety is higher, and it will not increase the occurrence of adverse reactions after the use of medication.[作者简介]戴卉(1985-),女,本科,主治医师,研究方向为内分泌科。

抗雌激素类乳腺癌治疗药物氟维司群及其衍生物的合成及
工艺研究的开题报告
一、研究背景
乳腺癌是世界范围内女性最常见的癌症之一。
随着医学科技的不断进步,乳腺癌的治疗方式也在逐步更新。
目前,抗雌激素类药物已成为乳腺癌治疗的重要手段之一,其作用是通过抑制雌激素对肿瘤细胞的作用来达到治疗乳腺癌的目的。
氟维司群(Fulvestrant)是一种新型的抗雌激素类药物,已获得欧盟、美国、日本等多个国家
的上市批准,成为乳腺癌治疗的重要选择之一。
二、研究目的
本研究旨在探讨氟维司群及其衍生物的合成方法以及其工艺流程,为该类药物的研发和生产提供参考。
三、研究内容
1.氟维司群的合成方法研究:探讨氟维司群的合成方法,包括催化剂的选择、反应条件的优化以及中间体的合成等。
2.氟维司群衍生物的合成研究:对氟维司群的结构进行改进和改良,合成出一系列相关的化合物,通过对其结构与活性的相关性进行研究,探索更加活性和可行的药物。
3.工艺优化研究:通过对氟维司群及其衍生物合成过程中的反应条件和工艺流程进行系统的优化,提高合成效率和产品质量。
四、研究意义
本研究将为氟维司群及其衍生物的合成提供新方法和技术,为该类药物的研发和生产提供技术支持,为药物的结构修饰和改进提供理论依据,为开发更安全、有效、
低副作用的抗雌激素类药物提供参考。

α-呋喃丙烯酸酯类香料的合成
付明华;祝丽娣
【期刊名称】《黑龙江医药》
【年(卷),期】2003(016)003
【摘要】目的:通过化合得到酯类化合物;方法:两种化学物质在催化剂的作用下,反应得到的酯类化合物,此反应得到的化合物,可以提高产品的收率,结果:收率为51.2%;结论:该系列酯类香料具有香气,作为香料广泛用于防晒类化妆品.
【总页数】2页(P218-219)
【作者】付明华;祝丽娣
【作者单位】哈药集团制药总厂,150086;哈药集团制药总厂,150086
【正文语种】中文
【中图分类】TQ46
【相关文献】
1.α—呋喃丙烯酸酯类香料的合成 [J],
2.呋喃丙烯酸酯类香料的合成与研究 [J], 张维成;孙宝国;徐理阮
3.SO42-/ZrO2催化香料α-呋喃丙烯酸丁酯的合成 [J], 苏文莉;刘长春
4.呋喃类香料2—呋喃丙烯酸正丙酯的合成 [J], 王建国
5.α-呋喃丙烯酸酯类香料的合成及性能研究 [J], 姚立红;苏长安;齐立权;陈新;铁梅;夏阳
因版权原因,仅展示原文概要,查看原文内容请购买。

薄层荧光扫描法测定花生根中白藜芦醇的含量
韩小丽;张士真;呼亚旭;李明静
【期刊名称】《化学研究》
【年(卷),期】2010(021)005
【摘要】在硅胶G铝箔板上,以V (氯仿): (丙酮):V (甲酸)=8:1:0.2的溶液为展开剂,狭缝尺寸为3 mm×0.45 mm,检测波长为335 nm,采用薄层荧光扫描法测定花生根中白藜芦醇的含量.结果表明:白藜芦醇在21.6 ~108.0 ng范围内线性关系良好,回归方程为Y=-664.701+12.666 X,R = 0.996 9,花生根中白藜芦醇的含量为358.89 μg/g.
【总页数】3页(P88-89,96)
【作者】韩小丽;张士真;呼亚旭;李明静
【作者单位】河南省医药学校,河南,开封,475001;商丘市技师学院,河南,商
丘,476000;河南大学,化学化工学院,河南,开封,475004;河南大学,化学化工学院,河南,开封,475004
【正文语种】中文
【中图分类】O652.63
【相关文献】
1.薄层扫描法测定虎杖中白藜芦醇含量 [J], 赵瑞芝
2.薄层荧光扫描法测定葡萄酒中的白藜芦醇及其糖苷异构体 [J], 陈敏;舒友琴;何计国;戴蕴青
3.薄层荧光扫描法测定刺五加片中异秦皮啶的含量 [J], 陈波
4.薄层荧光扫描法测定花生茎中白藜芦醇的含量 [J], 韩小丽;邵鹏;李明静;刘绣华
5.牛蒡子药材中牛蒡苷含量的薄层荧光扫描法测定 [J], 王淑静;魏永巨
因版权原因,仅展示原文概要,查看原文内容请购买。

含氟姜黄素类似物的合成及其抑制酪氨酸酶活性研究韦星船;郑成;何浏;罗颖;赵文忠;林学镁【期刊名称】《广州化工》【年(卷),期】2017(045)008【摘要】以对氟苯乙酮与氟苯甲醛进行羟醛缩合反应,合成3种不对称含氟姜黄素类似物.对产物进行分离提纯,对合成物结构进行表征.以L-多巴为底物,测试合成物对酪氨酸酶的抑制作用.结果表明:3种不对称姜黄素类似物对酪氨酸酶的抑制效果均强于姜黄素.合成物1-(4-氟苯基)-3-(4-氟苯基)丙烯酮(A3)对酪氨酸酶的半数抑制浓度IC50为36.7 μmol/L,其抑制作用是姜黄素(IC50 =95.7 μmol/L)的2.6倍.探讨了合成物抑制酪氨酸酶活性的构效关系.【总页数】3页(P61-63)【作者】韦星船;郑成;何浏;罗颖;赵文忠;林学镁【作者单位】广州大学化学化工学院,广东广州 510006;广州大学化学化工学院,广东广州 510006;广州大学化学化工学院,广东广州 510006;广州大学化学化工学院,广东广州 510006;广东拉芳日化有限公司,广东汕头515041;广东拉芳日化有限公司,广东汕头515041【正文语种】中文【中图分类】TS202.3【相关文献】1.2-茚酮基姜黄素类似物对酪氨酸酶的抑制作用 [J], 韦星船;缪绸雨;段彦飞;陈永洲;黄晓罗;张晓琳2.含氟姜黄素类似物的合成及其光谱分析 [J], 韦星船;刘自力;杜志云;郑希;张焜;何雄3.含氮姜黄素类似物的合成及其酪氨酸酶抑制效应 [J], 韦星船;霍梦月;郑成;段彦飞;杨前程;蔡伟平4.姜黄素多酚类似物对酪氨酸酶抑制活性的研究 [J], 涂增清;杜志云;张焜;潘文龙;汤志恺;莫容清5.新型单羰基姜黄素类似物的合成及抗肿瘤活性研究 [J], 周代营;田宇光;杜志云;赵肃清;郑希;张焜因版权原因,仅展示原文概要,查看原文内容请购买。

α-生育酚琥珀酸酯和三氧化二砷对阿霉素诱导的白血病细胞耐药性的预防作用刘秀华;魏虎来【期刊名称】《兰州大学学报(医学版)》【年(卷),期】2006(32)2【摘要】目的观察α-生育酚琥珀酸酯(α-TOS)和As2O3对阿霉素(ADM)诱发的向血病细胞耐药的预防作用.方法以白血病K562细胞为模型,模拟临床化疗过程,采用间歇性给药、逐渐增量的方法诱导K562细胞对ADM产生耐药性;同时并用α-TOS或As2O3,以观察其对ADM诱导耐药性产生的影响.MTT法检测细胞耐药性,免疫荧光法观察P-糖蛋白(P-gp)表达,荧光显微镜观察细胞内ADM含量.结果经ADM诱导5个月后,K562细胞对ADM的耐受性增高约4倍,并与柔红霉素交叉耐药,P-gp表达增加,细胞内ADM的蓄积量降低;同时并用α-TOS或As2O3可不同程度地降低ADM诱导的K562细胞P-gp的表达水平和增加细胞内ADM含量,减低或延缓耐药性发生.结论α-TOS和As2O3可降低或延缓ADM诱导的白血病细胞耐药性的发生,其机制可能为抑制P-gp的表达.【总页数】4页(P19-21,25)【作者】刘秀华;魏虎来【作者单位】兰州大学第二医院药剂科,甘肃,兰州,730030;兰州大学医学实验中心,甘肃,兰州,730000【正文语种】中文【中图分类】R969.2【相关文献】1.α-生育酚琥珀酸酯对过度运动小鼠NK细胞平衡及ApoE基因表达影响研究 [J], 王凤华;孔海军;孔令生;黄玲;李赵越2.α-生育酚琥珀酸酯抗小鼠Ehrlich氏腹水癌作用的研究 [J], 李红卫;吴坤3.α-生育酚琥珀酸酯对S180荷瘤小鼠抗肿瘤作用的实验研究 [J], 李红卫;苏怡;王玉燕;吴坤4.α-生育酚琥珀酸酯对急性放射病小鼠早期造血损伤的防护作用观察 [J], 贾超;张刘珍;邢爽;柳晓兰;善亚君;丛悦;崔宇;王丽梅;从玉文5.α-生育酚琥珀酸酯诱导人胃癌SGC-7901细胞凋亡及其作用的分子形式 [J], 李红卫;赵岚;吴坤因版权原因,仅展示原文概要,查看原文内容请购买。

醛氢叶酸、5-氟尿嘧啶、丝裂霉素联合应用治疗晚期食管癌陈兴田;陈希云;沈玉法
【期刊名称】《现代消化及介入诊疗》
【年(卷),期】1997(002)003
【摘要】5-氟尿嘧啶(5-Fu)、丝裂霉素(Mmc)为食管癌的主要化疗药物,单一用药及联合化疗,疗效不满意,根据提高细胞内还原叶酸含量能抑制胸腺嘧啶脱氧核苷酸合成酶,增强5-Fu细胞毒性作用的理论。
我们自1991年来设计5-Fu、Mmc和醛氢叶酸(FA)联合应用治疗晚期食管癌,并与单用5-Fu、Mmc作对照.现将结果报告如下。
【总页数】2页(P247-248)
【作者】陈兴田;陈希云;沈玉法
【作者单位】276400,山东省沂水中心医院消化内科;276400,山东省沂水中心医院消化内科;276400,山东省沂水中心医院消化内科
【正文语种】中文
【中图分类】R73
【相关文献】
1.奥沙利铂联合5-氟尿嘧啶、醛氢叶酸钙治疗晚期食管癌 [J], 姜虹;陆长虹;王理伟;周英;高宇
2.醛氢叶酸加氟尿嘧啶持续输注联合顺铂治疗晚期食管癌病人的观察与护理 [J], 陈嘉;江敏霞
3.醛氢叶酸加氟尿嘧啶持续输注联合顺铂治疗晚期食管癌病人的观察与护理 [J],
陈嘉;江敏霞
4.洛铂联合醛氢叶酸、氟尿嘧啶治疗晚期食管癌的临床研究 [J], 杨柳青;王宁菊;秦叔逵;钱军;马胜林;熊建萍;张映红;王华庆;张贺龙;蒋芹
5.艾恒、丝裂霉素、氟尿嘧啶辅以醛氢叶酸联合化疗治疗转移性胃癌的临床研究[J], 王文秀;徐玉清;姜秋颖;赫文;于常华;黄大勇;路丹;杨宇
因版权原因,仅展示原文概要,查看原文内容请购买。

二氢杨梅素及其酰化衍生物对肝细胞氧化损伤的保护作用
杜宝双;陈尚卫;李玥;朱松
【期刊名称】《食品与机械》
【年(卷),期】2022(38)6
【摘要】目的:考察二氢杨梅素(DHM)及其衍生物对肝细胞氧化损伤的保护作用。
方法:建立L02细胞过氧化氢损伤模型,通过酶法酰化修饰DHM得到不同亲脂性的DHM衍生物,再通过测定活性氧(ROS)、乳酸脱氢酶(LDH)、丙二醛(MDA)、过氧
化氢酶(CAT)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-PX)水平、线粒体膜电位和Caspase 3水平评估DHM及其衍生物对肝损伤细胞的保护作用。
结果:除3-O-月桂酰化二氢杨梅素外,其余衍生物处理后的肝损伤细胞中活性氧(ROS)、乳酸脱氢酶(LDH)的释放和丙二醛(MDA)水平显著降低,抗氧化酶系水平升高,线粒
体膜电位升高,Caspase 3水平降低,其中3-O-辛酰化二氢杨梅素的保护效果最好。
结论:中等链长二氢杨梅素衍生物有较好的护肝效果。
【总页数】5页(P30-33)
【作者】杜宝双;陈尚卫;李玥;朱松
【作者单位】江南大学食品科学与技术国家重点实验室;江南大学食品学院
【正文语种】中文
【中图分类】S66
【相关文献】
1.二氢杨梅素对糖耐量异常大鼠肾脏氧化损伤的保护作用
2.二氢杨梅素对肝纤维化大鼠脂质过氧化损伤的保护作用
3.二氢杨梅素对APAP诱导小鼠急性肝损伤的保护作用
4.二氢杨梅素对H2O2诱导PC12细胞损伤的保护作用研究
5.二氢杨梅素对心脑血管疾病损伤保护作用的研究进展
因版权原因,仅展示原文概要,查看原文内容请购买。