单冻虾仁HACCP计划书
- 格式:docx
- 大小:53.08 KB
- 文档页数:37
实训项目4:食品企业HACCP计划的构建分项目1 产品特性与预期用途的描述分项目2 冻煮小龙虾仁生产加工工艺流程图的编制、确认和控制1、冻煮小龙虾仁生产工艺流程图原料虾验收↓原料虾挑选↓运输清洗↑↓冷藏蒸煮↑↓装箱冷却↑↓冻结包装材料去头、去壳、去黄、↑↓去肠、分级真空装袋验收↓↑↓半成品验收→称重、装袋←储存2、冻煮小龙虾仁生产工艺描述原料虾验收:原料的好坏直接影响到成品质量的好坏,因此,原料验收与挑选是很重要的一环。
供应商必须提供产地证明及标签;经检验合格后验收。
验收原料时首先要观察整批虾体的清洁程度并嗅其气味,一般在较为清洁卫生的环境中生长的小龙虾,虾体色泽鲜艳、洁净,嗅之无异味。
反之,生长在农田、死水沟里的龙虾,其虾体表面污秽不洁,且很难洗净,嗅之有污泥味,甚至有农药或其它异味,此种虾应拒绝收购。
严格挑出死虾及老壳虾。
原料虾挑选:严格挑出死虾、病虾和老壳虾,以及小虾和不符合规格大小的虾。
原料虾清洗:用清水喷淋清洗3分钟,水温不高于10℃并且,个别不易清洗的应用刷子进行刷洗。
蒸煮:蒸煮在100℃开水中进行,蒸煮时间的长短应视不同季节、虾壳的不同厚度、虾体的大小等来决定。
蒸煮时间过短,会造成杀菌不彻底;而蒸煮时间过长,会造成出品率降低,虾仁弹性及口感变差。
冷却:自来水预冷及冰水冷却的目的是使蒸煮的龙虾立即降温,以达到保证虾仁品质在加工过程中不发生改变的目的。
预冷冷却用水应在使用前化验检测,若在微生物指标上未达到生活饮用水的标准,可加适量的含氯消毒液(如二氯异氰尿酸钠、三氯异氰尿酸钠等)使其达到标准,并在使用过程中及时更换,保证预冷冷却用水不对虾体造成新的污染。
冷却水温度应达到2℃~4℃,预冷冷却间的空气应基本无菌。
去头、去壳、去黄、去肠、分级:在操作台上用手工去头、壳、黄、肠。
不得有未抽尽的肠腺,虾仁需完整,更不能有杂质。
去净肠腺及虾黄时,应防止肠腺断裂,以免残留断肠及污染虾肉(煮熟后的虾肠、脾内容物含大肠菌群高达240个/g~460个/g).另外,虾黄去净与否对虾肉成品质量影响很大。



目录1.1 颁布令1.2 前言1.3 HACCP小组成员及分工1.4 产品描述1.5 加工工艺流程图1.6 加工工艺描述1.7 危害分析工作单1.8 HACCP计划表1.9 HACCP 计划制订程序2.0 CCP操作控制验证程序2.1 HACCP 计划验证程序2.2 CCP点记录管理程序1.1、总经理颁布令三亚升达水产有限公司的《南美白对虾HACCP计划》依据美国FDA的HACCP法规要求,结合我国的实际需要而制定的。
它的主要目的是:通过危害分析和建立关键控制点,在生产加工南美白对虾过程中,控制、降低和消除生物的、化学的、物理的显著危害,确保产品的安全卫生。
经审定,此HACCP计划切实可行,可以满足消费者对食品安全卫生的需求,公司全体员工必须遵照执行。
现予以批准发布,并定于二OO三年七月八日实施。
总经理:二OO六年九月二十日1.2 .1前言水产品加工过程实施系统和有效的卫生质量控制,是保障水产品加工产品质量的关键,HACCP(Hazard A nalysis and Critical Control Point )的缩写,即危害分析关键控制点的管理程序,是目前被广泛用于水产品加工过程卫生质量监控体系,且其实用性也实践证明了这一项质量系统控制技术。
我公司根据HACCP的原理,结合(南美白对虾)工艺的特点,制订了本工厂HACCP管理程序手册,以此作为去头虾加工过程中建立HACCP监控系统,实施HACCP 管理程序的工作指导书。
1.2.2、企业背景材料三亚升达水产有限公司始建于2000年8月,2000年11月正式投产,是一家私营股份制企业,是以生产南美白对虾、鱿鱼、带鱼,大目莲、水产品为主的企业,公司占地面积为2000多平方米,建筑面积1600多平方米,固定资产880万元,拥有职工70人,各类技术人员12人,有先进的速冻平板机,单冻库、平板机1条生产线,年产量3600吨,其中生产虾产品400吨,鱿鱼150吨,带鱼300吨,大目莲、马头鱼等150吨。

单冻虾HACCP计划一、HACCP计划的目的单冻虾HACCP计划的目的是确保单冻虾的安全可食用性,以及确保单冻虾的质量和品质。
二、HACCP计划的范围本HACCP计划所涉及的范围包括从单冻虾的采摘到出厂的全部生产过程,包括:采摘、清洗、蒸煮、冷却、分割、包装、贮藏及运输等。
三、HACCP计划的原则1. 危害分析:确定可能存在的安全危害,确定控制点,并采取有效的措施来控制和预防这些危害。
2. 重点控制点:确定可能对单冻虾安全可食用性产生影响的重点控制点,并采取有效的措施来控制和预防这些危害。
3. 标准设定:确定每一个控制点的标准,以确保单冻虾的安全可食用性。
4. 监测:定期监测每一个控制点,以确保单冻虾的安全可食用性。
5. 文件记录:对每一个控制点的监测结果和控制措施进行记录,以便日后查阅。
6. 响应措施:如果发现单冻虾存在安全危害,应立即采取有效的措施来控制和预防这些危害。
四、HACCP计划的详细内容1. 采摘:(1)采摘的虾应当符合当地的食品安全标准;(2)采摘前应进行水质检测,确保水质符合食品安全标准;(3)采摘时应使用清洁的采摘工具,并保证虾的洁净;(4)采摘后应立即进行清洗,以确保虾的新鲜度。
2. 清洗:(1)清洗时应使用清洁的清洗工具;(2)清洗时应使用清洁的水,并保证水温不超过50℃;(3)清洗后应立即进行蒸煮,以确保虾的新鲜度。
3. 蒸煮:(1)蒸煮时应使用清洁的蒸煮工具;(2)蒸煮时应保证蒸煮温度不低于70℃,蒸煮时间不少于3分钟;(3)蒸煮后应立即进行冷却,以确保虾的新鲜度。
4. 冷却:(1)冷却时应使用清洁的冷却工具;(2)冷却时应保证虾的温度不超过2℃;(3)冷却后应立即进行分割,以确保虾的新鲜度。
5. 分割:(1)分割时应使用清洁的分割工具;(2)分割时应保证虾的温度不超过2℃;(3)分割后应立即进行包装,以确保虾的新鲜度。
6. 包装:(1)包装时应使用清洁的包装工具;(2)包装时应保证虾的温度不超过2℃;(3)包装后应立即进行贮藏,以确保虾的新鲜度。
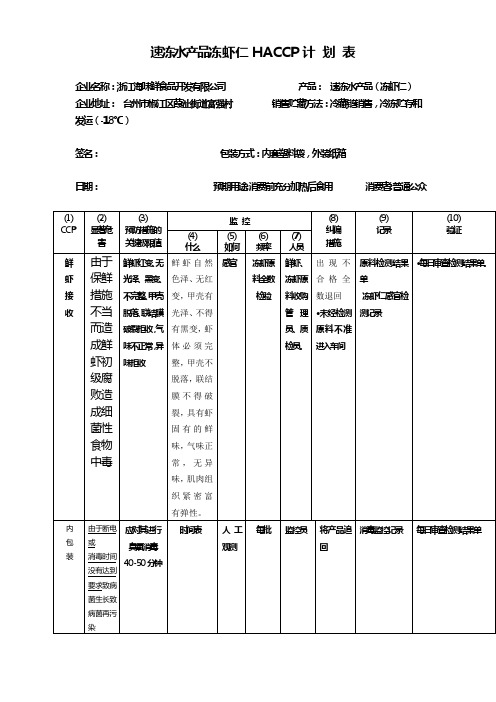
速冻水产品冻虾仁HACCP计划表企业名称:浙江海味鲜食品开发有限公司产品:速冻水产品(冻虾仁)企业地址:台州市椒江区葭沚街道富强村销售贮藏方法:冷藏链销售,冷冻贮存和发运(-18℃)签名:包装方式:内套塑料袋,外装纸箱日期:预期用途:消费前充分加热后食用消费者:普通公众(1) CCP(2)显著危害(3)预防措施的关键极限值监控(8)纠偏措施(9)记录(10)验证(4)什么(5)如何(6)频率(7)人员鲜虾接收由于保鲜措施不当而造成鲜虾初级腐败造成细菌性食物中毒鲜虾红变、无光泽、黑变、不完整、甲壳脱落、联结膜破裂拒收,气味不正常,异味拒收鲜虾自然色泽、无红变,甲壳有光泽、不得有黑变,虾体必须完整,甲壳不脱落,联结膜不得破裂,具有虾固有的鲜味,气味正常,无异味,肌肉组织紧密富有弹性。
感官冻虾原料全数检验鲜虾、冻虾原料收购管理员、质检员。
出现不合格全数退回〃未经检测原料不准进入车间原料检测结果单冻虾仁感官检测记录〃每日审查检测结果单。
内包装由于断电或消毒时间没有达到要求致病菌生长致病菌再污染应对其进行臭氧消毒40-50分钟时间表人工观测每批监控员将产品追回消毒监控记录每日审查检测结果单危害分析表公司名称:浙江海味鲜食品开发有限公司产品描述:速冻水产品(冻虾仁)地址:浙江销售贮藏方法:冷藏链销售;冷冻贮存和发运(-18℃)签名:包装方式:内套塑料袋,外装纸箱日期:预期用途:消费前充分加热后食用消费者:普通公众(1) (2)(3)(4)(5)(6)加工工序识别本工序被引入、控制或增加的潜在危害潜在食品危害是否显著对第3栏的判定依据能用于显著危害的预防措施是什么?该步骤是关键控制点吗?(是/否)(1) 鲜虾接收生物的致病菌、非致病菌[放线菌科—链霉菌属—委内瑞拉链丝(霉)菌产生氯霉素(CM)]是鲜虾产自东海海域,海洋存在大量微生物,以及环境致病菌的存在,虾可能受到致病菌的污染,人们食用后致病。
鲜虾捕捞后需充分加冰保鲜,控制不当有可能引起微生物扩增。


单冻虾仁HACCP计划书一、食品安全计划为确保单冻虾仁质量安全,我们将建立和实施全面的食品安全计划。
本食品安全计划是基于危害分析和关键控制点原则(HACCP)的食品安全管理系统。
我们将定期评估食品安全计划的有效性,并根据评估结果进行必要的调整和改进。
二、制造过程描述我们的单冻虾仁的制造过程如下:1. 处理:原料虾仁进厂后,通过清洗、生煮、析壳、洗净等工艺,去除虾壳、虾线、虾头等杂质,得到干净的虾仁。
2. 装袋、称重:虾仁装入塑料袋中,并按照规定重量称重。
3. 冷冻:虾仁经过快速冷冻,使其温度降到-18℃以下。
4. 包装、存储:冷冻虾仁包装在塑料袋中,并在-18℃以下的冷库中存储,等待出货。
三、危害分析通过对制造过程进行分析,我们确定了以下可能导致食品安全危害的因素:1. 微生物:细菌、霉菌、病毒等。
2. 化学物质:重金属、农药、添加剂等。
3. 物理因素:碎片、玻璃等。
四、关键控制点我们确定了以下关键控制点(CCP):1. 处理环节:在洗净虾仁、生煮和析壳等环节中,必须采取严格的卫生措施,确保原料的新鲜和卫生。
2. 冷冻环节:在快速冷冻的过程中,必须确保温度达到-18℃以下,并保持该温度,以避免微生物繁殖。
3. 包装和存储:在包装和存储过程中,必须确保塑料袋的完整性,以避免物理污染;并确保冷库温度始终低于-18℃,以避免微生物繁殖。
五、监测我们将对以上三个关键控制点进行监测,并记录监测数据,以确保制造过程中的食品安全。
1. 处理环节:我们将记录原料虾仁的生产日期、产地、入厂日期和批次号等信息,以便进行追溯;并进行卫生检测,检验原料虾仁是否符合卫生标准。
2. 冷冻环节:我们将对冷藏设施进行定期检查,检查温度计是否准确,并记录温度数据以及冷冻时间,以确保温度是否达到-18℃以下,并符合规定的冷冻时间。
3. 包装和存储:我们将在包装过程中对塑料袋进行检测,检查塑料袋是否完整;并定期检查冷库的温度计和湿度计,以确保温度和湿度符合标准要求。

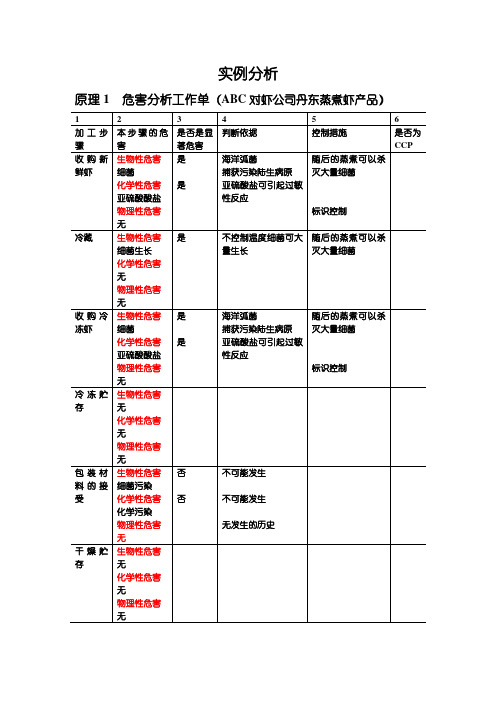
实例分析
原理1 危害分析工作单(ABC对虾公司丹东蒸煮虾产品)
原理2 确定关键控制点
关键控制点决定树(判断结果示意表)
原理3 建立关键限值(临界限)
原理4 确定关键控制点的监控措施
原理5 确定校正措施
原理6 菌落验证程序证实HACCP系统的有效性HACCP计划表
原理7 确定有效的记录的保持程序
应包含的内容
1.原料评价单记录检查接受的原料虾重是否有亚硫酸盐,记录供
应商的信息
2.供应商的保证函
3.虾蒸煮的记录记录时间和温度
4.包装间检查记录记录用亚硫酸盐处理的虾是否被适当的标记附加记录:
1.实验室结果亚硫酸盐残留
2.蒸煮工艺确认书
3.蒸煮设备确认书
4.设备校准表
5.实验室报告
6.纠正措施记录范例
7.员工培训记录。


单冻虾仁H A C C P 计划书 HC/02-2002 2002-5-15 实施法人代表: 电话:0086-0 传真:0086- 0 本计划书由质技部编印2002-4-10 发布名称:水产有限公司地址:邮编:316101E-MAIL:sB 、单冻生虾仁工艺流程图18.包装材料贮藏20. 装外纸箱 21. 冻 藏3.冻虾原料CCP11. I 鲜虾接收CC 甬 19.包装/贴标CCP222. 出厂发运注:泡药工序根据客户要求。
SSOP计划书卫生标准操作程序(2002年第一版)企业名称:企业地址:关于实施2002年SS0P计划的发布令2002年5月份,公司HACCP办公室根据中国《出口食品生产企业卫生要求(GMP)》、国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》的要求,并参考了美国FDA21CFR第110部分一-食品生产的现行良好操作规范的规定,制定了第一版SS0P计划书,请你们严格按照本计划书认真执行。
做到从原料到成品由专人负责,严格把关,不断健全和完善食品安全预防控制体系(主要指卫生管理体系),确保产品在符合国际食品法典委员会规范及中国《出口食品生产企业卫生要求(GMP)》的要求基础上进行加工。
本颁布令从2002年5月16日起开始实施。
本公司各有关部门、各生产岗位管理人员和具体操作员工均需毫无例外地执行此颁布令。
总经理(兼HACCP办公室主任):2002年5月15日一. 生产用水和冰的安全二. 与食品接触表面的卫生情况三. 防止交叉污染四. 洗手、消毒和更衣室、厕所设施的卫生五. 防止食品被污染六. 有毒化合物的管理使用七. 人员卫生的管理八. 害虫的防止注:GMP良好操作规范检查按照中国《出口食品生产企业卫生要求》、国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》的要求,并参考了美国FDA21CFR 第110部分食品生产的现行良好操作规范的规定,结合本公司的情况制定相应的SS0P计划如下:一. 生产用水和冰的安全1、目标:直接接触食品,食品接触表面和用于制冰的水来自安全卫生的水源或经处理后达到要求的水源。

SEAFOOD HACCP ENCORE COURSESEAFOOD HACCP ALLIANCESEAFOOD HACCP ENCORE COURSEINTRODUCTIONThe Food and Drug Administration made significant changes to the rules regulating the safe and sanitary processing of fish and fishery products, including imported seafood, in December 1997 (the implementation date for the Seafood HACCP Regulation, 21 CFR 123). The regulations mandate the application of Hazard Analysis Critical Control Point (HACCP) principles to the processing of seafood. Since the implementation date, many processors have successfully developed and implemented HACCP programs to control food safety hazards associated with their products and processes. Additionally, many processors have developed and implemented successful sanitation monitoring programs, for the eight sanitation elements, that are required as part of the new regulation. While many have been successful in complying with the requirements of the regulation (both HACCP and sanitation monitoring), there remains a large segment of the industry for which compliance has been difficult. The Encore HACCP Training Program is designed to assist those processors who are experiencing difficulty in complying with the regulation. Moreover, it is intended to give regulatory personnel a more thorough understanding of HACCP to assist them in evaluating the adequacy of industry developed HACCP plans.The National Seafood HACCP AllianceThe Seafood HACCP Alliance was founded through a project funded by the National Sea Grant College Program to develop a standard training and education program to assist the commercial and regulatory implementation of FDA's mandatory seafood HACCP inspection program. The Seafood HACCP Alliance Steering Committee includes representatives from three federal programs (FDA, USDA's Cooperative State Research, Education and Extension Service, and the National Marine Fisheries Service), state regulatory agencies through the Association of Food and Drug Officials (AFDO), the Interstate Shellfish Sanitation Conference, industry trade associations (National Fisheries Institute and the National Food Processors Association), and university faculty from extension and Sea Grant programs. Current funding for the Alliance is being shared by the National Sea Grant College Program, AFDO, and FDA. Examples of Current ProblemsSince implementation of the new regulation in December 1997, FDA has issued "untitled letters" and "warning letters" to processors citing deficiencies in their HACCPplan and/or the sanitation monitoring program. Problems typically include:! When a corrective action is listed in the HACCP plan, it often does not meet the requirements of 21 CFR 123.7(b), because it either: 1) does not adequatelyensure that adulterated product does not enter the market; or 2) does not correct the cause of the deviation. For example, sensory analysis is not a suitablecorrective action when time/temperature critical limits have been breached in the control of histamine. On the other hand, adjusting the cooler temperature in sucha situation does not address the safety of the product exposed to the adversecondition.! The distinction between which hazards must be addressed by the primary processor only (e.g., ciguatera, pesticides, aquaculture drugs) and those which must be addressed by any processor (e.g., histamine, pathogens, foodadditives) is apparently not clear.! Where time/temperature controls are necessary to prevent a food safety hazard(e.g., exposure time during processing, cooler temperature, or presence of ice toprevent pathogen growth or histamine formation), the controls must be included in the HACCP plan, rather than controlled through application of GMPs or anSSOP.! The linkage between the critical limit and the monitoring procedures is often weak. For example, monitoring cooler temperature is not an appropriate means of ensuring that a cooked product is cooled to the prescribed internal producttemperature within the prescribed time period (i.e., critical limits), unless a study has been performed to relate the two under the least favorable processingconditions that are likely to occur.! Setting a critical limit for maximum cooler temperature in which product is consistently stored iced has created a number of problems. Because theproduct is iced, processors often will not take a corrective action. However, this results in them deviating from their prescribed corrective action plans. A moreappropriate scenario would be to prescribe corrective action only when: 1) thecooler temperature exceeds the limit and the product is not adequately iced; or,2) the cooler temperature exceeds the limit and the product temperatureexceeds a second limit.! There must be scientific support for all critical limits selected by a processor(e.g., the time and temperature of a cooking process, the length of exposure of atemperature-sensitive product), and the support must be relevant to theprocessing conditions present in the facility. One source of support is FDA’sHazards and Controls Guide (subsequently referred to as the Guide).The following table summarizes some of the common compliance issues documented in recent warning letters issued by FDA.HACCP Compliance IssuesSummary of Common Compliance ProblemsNo written HACCP plan when one is neededHazard not listed in planAppropriate critical limits not listedAdequate monitoring procedures not listedMonitoring Procedures not followedCorrective action in plan not adequateInadequate sanitation monitoringInadequate sanitation monitoring recordsSummary of Significant Hazards Not Listed In HACCP PlansCiguateraHistamineClostridium botulinum toxinSummary of Significant Hazards Not Adequately ControlledCiguateraHistamineClostridium botulinum toxinPathogens survival through cookingSulfitesCourse AgendaModel Course Agenda1 -Introduction and Background2 -Module 1: Performing a Hazard Analysis for Fresh/Frozen Finish! FDA video presentation on conducting a Hazard Analysis! Practical: Hazard Analysis and Discussion3 -Module 2: Performing a Hazard Analysis for Cooked RTE Crustaceans! FDA video presentation on conducting a Hazard Analysis! Practical: Hazard Analysis and Discussion4 -Module 3: Performing a Hazard Analysis for Smoked Fish (optional)! FDA video presentation on conducting a Hazard Analysis! Practical: Hazard Analysis and Discussion5 -Module 4: Developing a HACCP Plan for Fresh and Frozen Finfish! FDA video presentation on developing a HACCP Plan! Questions/Answers6 -Module 5: Developing a HACCP Plan for Cooked RTE Crustaceans! FDA video presentation on developing a HACCP Plan! Questions/Answers7 -Module 6: Developing a HACCP Plan for Smoked Fish (optional)! FDA presentation on developing a HACCP Plan! Questions/Answers8 -Practical: Developing a HACCP Plan! Participants (grouped in small teams) will develop a HACCP Plan.All groups can work on the same species and processes, or at thetrainers discretion, chose different species and processes.! Discussion of participant-developed HACCP Plans9 -Module 7: Sanitation Monitoring! FDA video presentation on sanitation monitoring! Practical exercise and discussion10. Course Wrap-upAlthough a nationally consistent training course is important, relevance of the training to the participants is even more important. Consequently, a certain level of flexibility among training programs is expected. The opportunity for trainers to customize training to the needs of their audience is provided in two areas. First, it is expected that subsequent to the training videos and discussions, the trainer will break the participants into small groups (HACCP teams) and each team will then work on a Hazard Analysis and HACCP Plan for selected species/products. Thespecies/products chosen is left to the preferences of the instructors and participants. The second area for added flexibility is the decision to include the Smoked Fish example into the training curriculum. The Alliance has included a smoked fish model as part of the Encore Training. However, in certain regions of the country where smoked fish processing does not exist, it may not be appropriate to include this product as part of the training. In its place, instructors have the option of including a model that is relevant to the needs of the audience, or expand the discussions on the previousmodels (finfish or cooked ready-to-eat crustacean), or spend more time on the sanitation module. While the use of the smoked fish model is optional, the other components of the training are not. In order to assure training conformity the following agenda is recommended:Understanding the AudienceIn order to make the Encore Training meaningful, it is essential to know the issues that are of importance to the participants. Experienced instructors use various means to arrive at some level of understanding of their audience. One simple, but effective approach is to ask participants to introduce themselves and to tell everyone what types of products their companies produce. Obviously, this should be done at the very beginning of the course. This information is helpful in determining what issues need to be given emphasis as well as those that do not. For example, if no one processes smoked fish, the instructor may be wise not to use those modules in the course, and thus save time to focus on other areas. Also, if no one processes histamine-susceptible species then it may not be useful to dwell on issues associated with this hazard. Conversely, if processors of histamine-susceptible species are present, it would be prudent to assure that points relevant to this hazard receive emphasis. Finally, it is important to direct participants to additional sources of information or assistance available in the region (e.g., extension and sea grant specialist as well as FDA district offices).Training MaterialsA manual for the Encore course has been developed and is available from the Alliance Web Site at the University of California at Davis(/haccp/ha.htm) and through FDA's Web Site(). Instructors may choose to download the manual and provide copies to the course participants, or notify participants of the web site's address and suggest that they download and print a hard copy to bring to the course. This manual will contain the text of the instructional videos, as well as the graphics and course introduction. For the practical exercises, it is recommended that instructors use material from the three-day Basic Seafood HACCP Course. Participants are expected to bring the following materials to the Encore Course:• The Seafood HACCP Alliance Training Curriculum Manual (from three-day basic course)• The FDA Fish and Fishery Products Hazards and Controls Guide• Copy of the firm’s HACCP Plan (including process flow diagram, hazard analysis (ifavailable)1• Copy of the firm’s SSOP (if available)1Models in Seafood HACCP Training Curriculum vs. Encore CourseAlliance training has traditionally underscored the dynamic nature of HACCP. Good examples of this are some differences in the expectations in the models provided in the Seafood HACCP Alliance Training Curriculum Manual and those discussed in FDA's video presentations. The models in the Curriculum Manual may be more rigorous than what FDA now considers reasonable to control certain hazards.FDA's Hazards and Controls GuideIt should be noted that the Encore training relies heavily on the advice contained in FDA’s Hazards and Controls Guide. This is intentional since the purpose of the training is to improve the level of compliance with the HACCP regulation, and the most direct way to assure compliance is to follow the guide.The Guide contains FDA's best advice on those hazards that are reasonably likely to occur and the controls that are necessary to address them. The Guide contains a lot more information than can be provided in this short course format, so you should review it carefully on your own.It is important that you understand that the material in the Guide is guidance only. It is not regulation. The Guide contains useful information on how to perform a hazard analysis and develop a HACCP plan. You can choose to perform your hazard analysis or control your hazards in a way that is different from what is outlined in the Guide, but understand that if you do, the burden is on you to demonstrate that you are providing an equivalent level of control.To demonstrate an equivalent level of control may not be easy. It will always require some sort of scientific information - maybe a study, maybe a search of the literature. These efforts may be burdensome and, in most cases, it is unlikely that processors will choose to take on that burden.1These items could serve as the basis for discussion and examples if the participants are comfortable in sharing the information with the class.This course is designed in part to make you familiar with the material contained in the Guide. Under most circumstances, if you follow that guidance you will have a HACCP plan that is acceptable to FDA.Furthermore, some of the advice in the Guide is currently the subject of scientific discussion and debate. As these issues are resolved, changes in FDA recommendations may be reflected in subsequent editions of the Guide.During the course, you may notice that a few shortcuts are taken from the process explained in the Guide. That was done to fit this material into the tight timeframes of a one-day course. But, you should use the formal HACCP process. It may save you from making some mistakes.MODULE 1PERFORMING A HAZARD ANALYSISPROCESSOR/DISTRIBUTOR OF FRESH/FROZEN FINFISH (EXCLUDING SMOKED, COOKED, DRIED, SALTED, PICKLED, BREADED FISH)This first module covers performing a hazard analysis for processors and distributors of raw, fresh and frozen finfish. It does not cover smoked, cooked, dried, salted, pickled, or breaded fish. Some of these products will be covered in later modules.There are a few steps that should perform at the beginning of the hazard analysis. They will help you through the process of doing your hazard analysis. It may seem pointless to spend the time to develop this information, but often processors who skip this step, make mistakes later on in the hazard analysis.You may use the checklist at the end of this module to keep this information together and as a quick reference.• List all of the species of fresh or frozen finfish that you handle. Often processors of this type handle a long list of species.• Identify those species which are purchased:Ø directly from the fisherman;Ø directly from the grower;Ø from another processor;Ø from a combination of these sources.• Identify how the fish are received:Ø fresh - under refrigeration;Ø fresh - under ice or chemical refrigerant;Ø frozen;Ø more than one of these methods.• Identify how the fish are stored after receipt:Ø fresh - under refrigeration;Ø fresh - under ice;Ø frozen;Ø more than one of these methods.• Identify how the finished product will be shipped:Ø fresh - under refrigeration;Ø fresh - under ice or chemical refrigerant;Ø frozen;Ø more than one of these methods.• Identify how the finished product will be packaged:Ø Traditional air-permeable packaging;Ø reduced oxygen packaging, such as: vacuum packaging, modified atmosphere packaging, controlled atmosphere packaging; hermetically sealed packages; or packed in oil.• Identify those which are intended to be consumed:Ø raw;Ø cooked;Ø both raw and cooked.If you put all this information on the checklist it could look like the completed example at the end of this module. One of the fish that this hypothetical firm handles is salmon. You can see that they receive both direct from the fishermen and direct from the grower. In both cases the fish are received fresh – refrigerated. They are stored fresh - refrigerated upon receipt, and the finished product is shipped both fresh - refrigerated and frozen. All products are packed in traditional air-permeable packaging and are intended to be cooked by the consumer.Remember from your basic HACCP course, it is advisable to create a flow diagram for your product, where you identify each of the processing and storage steps. We are not going to cover that procedure here, except to say that you may find it useful to indicate next to each processing step on the flow diagram, the maximum length of time that the product could be exposed to unrefrigerated temperatures, and the maximum room air temperature, or, if you know it, the maximum internal product temperature, during that processing step. This will help you calculate maximum cumulative exposure time, something that’s important for a variety of products. We will cover that in detail in a later module.Species Related HazardsThis information, and the information in the checklist will help you perform the next two steps of the hazard analysis: brainstorming the potential hazards (i.e. hazard identification) and determining whether or not they are significant (i.e. hazard evaluation). Consider the list of hazards that might apply.Natural ToxinsFirst is natural toxins. For finfish, these include: ciguatera, or CFP; amnesic shellfish poisoning, or ASP; and paralytic shellfish poisoning, or PSP. Although there are some differences, we'll cover them together. Ciguatera is a toxin that is associated with certain subtropical and tropical reef fish. ASP and PSP are toxins that are most commonly associated with molluscan shellfish, but can also be present in some finfish species.To determine if the species you handle have a potential natural toxin hazard, you can look for a mark in the fourth column of (page 12) 3-1 in the Guide. These species have been associated with ciguatera toxin poisonings or with ASP or PSP levels above FDA's action level.A natural toxin hazard is usually significant at the receiving step if all of these conditions are met:• you received the fish directly from the fisherman; and• there is an historical occurrence of the toxin in fish from any waters from which you receive fish; and• in the case of ASP and PSP only, the fish are marketed uneviscerated. This last point is important, because toxic levels of ASP and PSP have not been found in the flesh of fish.There is more on this subject in the Natural Toxins chapter of the Guide. Natural toxins in finfish is an example of a hazard that need only be controlled by the primary, or first, processor. Subsequent processors can rely on controls that are provided by the primary processor, and do not need to include controls for this hazard in their own HACCP plans.Environmental ChemicalsNext, is the hazard of environmental chemicals (e.g. pesticides). Like you did with natural toxins, go to Table 3-1 in the Guide, but this time look in the sixth column. If there is a check there, this is a potential hazard. Finfish species listed this way are harvested from fresh water and near-shore locations.The environmental chemicals hazard is usually significant at the receiving step if you receive the fish directly from the fisherman or grower, and either one of these conditions is also met:• there are permanent or occasional closures to commercial harvesting of finfish in any harvest area from which you receive fish; or• the fish are raised in ponds.Environmental chemicals is another example of a hazard that need only be addressed by the primary processor. Secondary processors do not need to include controls for this hazard in their HACCP plans.Aquaculture drugsNext is aquaculture drugs. Again, go to Table 3-1, and look in column seven for a check mark. Or, you can just assume that if the fish is aquacultured, you will have a potential aquaculture drug hazard.The aquaculture drug hazard is usually significant at the receiving step if you receive the fish directly from the grower - not if you receive it from another processor. In some regions and in some species (e.g. aquaculture of crayfish in the Gulf Coast region of the U.S.) aquaculture drugs are not used. In these cases, the hazard would not be significant. Aquaculture drugs is yet another example of a hazard that need only be controlled by the primary processor.ParasitesThe next hazard is parasites. This time look in Table 3-1 for a check mark in column 3. The marked species commonly contain infective parasites.The parasite hazard is usually significant if the product is intended to be consumed raw. But, there are exceptions. The hazard would not usually be significant if:• it is an aquacultured product that is raised on pelleted feed; or• it was received already frozen; or• you have obtained evidence that it will be frozen by a subsequent processor or institutional user.Even so, there is considerable scientific debate about the frequency of occurrence of fishborne parasitic infections. FDA is in the process of collecting data on this subject, both in the U.S. and overseas. So, FDA will defer regulatory action for failure to control this hazard until after the data is collected and evaluated. This is a topic that you should continue to monitor. If, based on the data, FDA changes its position on the significance of the parasite hazard, the new position will be reflected in the next edition of the Guide.HistamineHistamine is a toxin that develops when certain species of finfish are exposed totime/temperature abuse. For this hazard, look in table 3-1 for a check mark in the fifth column. A check mark means that the species has a potential histamine hazard.The histamine hazard is usually significant at the receiving step if:• you receive fish directly from the fisherman; or• if you receive the fish from another processor, unless the fish are received frozen. The histamine hazard is also usually significant during processing or storage if unsafe levels of histamine could form as a result of cumulative time/temperature abuse. You may already have procedures in place in your plant that prevent this type oftime/temperature abuse, such as: refrigeration, icing, or time/temperature management. To assess whether the histamine hazard is significant, you should consider the amount of time/temperature abuse that could occur if your existing procedures were not followed (e.g. if you did not use ice, refrigeration, or time/temperature management). If histamine could form under those conditions, then the hazard is significant.There are exceptions. The hazard is not usually significant if the fish remains frozen during storage or processing. Also, it may not be significant for fresh fish products, during processing steps that are brief, such as mechanical filleting.Recently, FDA updated the material in the Guide concerning histamine to provide information on the limits of safe exposure time/temperature. The new guidance limits are:• for previously frozen fish:• 24 hours as long as the fish are not exposed to temperatures above 70o F• 12 hours if the fish are exposed to temperatures above 70o F.• for fish that have not been frozen:• 8 hours as long as the fish are not exposed to temperatures above 70o F• 4 hours if the fish are exposed to temperatures above 70o F.Remember, these are FDA recommendations. Other time/temperature combinations may also be valid if they can be shown to be scientifically supportable.You apply the worst case unrefrigerated exposure times and temperatures you developed for each of the processing steps in the flow diagram for your product to these cumulative exposure limits. If the total is approaching the applicable safe exposure limit, you should usually consider the histamine hazard as significant for those processing steps. If not, the hazard is not likely to be significant.That covers the significant hazards associated with the species of fish. But, as you know, hazards are not only species-related, but are also process-related. Now consider the process-related hazards that apply to fresh and frozen finfish.Process-related HazardsTable 3-3 (page 41) outlines the potential process-related hazards for different kinds of finished product. Most finished product categories are broken down by the packaging type – air pack or reduced oxygen pack. The reduced oxygen packaging categories are further broken down by the method of finished product storage – refrigerated or frozen. In the categories for raw fish, which includes both finfish and non-finfish species, four potential hazards are identified. Only three of those apply to raw finfish: pathogen growth as a result of time/temperature abuse; Clostridium botulinum toxinformation; and metal fragments. The fourth, food and color additives, relates to the sulfite hazard in non-finfish species, which will be covered in the next module.Clostridium botulinumFirst is Clostridium botulinum. C. botulinum is a pathogen that grows in the absence of air and can produce a lethal toxin in the food. The table identifies C. botulinum as a potential hazard only for vacuum packaged, modified atmosphere packaged, and controlled atmosphere packaged fish, fish in hermetically sealed packages, and fish packed in oil - what are called reduced oxygen packaging.The hazard is usually significant for fish in these packaging types unless the product is:• packaged in an oxygen permeable package; or• immediately frozen after processing, maintained frozen throughout distribution, and labeled to be held frozen and to be thawed under refrigeration immediately before use.FDA is not currently aware of an effective control strategy for the C. botulinum hazard in fresh finfish in these packaging types, other than strict temperature control to ensure that the fish does not exceed 38o F long enough to produce toxin. This strategy is not practical once the product leaves your control. There has been well-documented temperature abuse in the food-service, retail and consumer sectors, that makes reliance on temperature as the only means of control, under ordinary circumstances, unreasonable. However, processors may be able to develop control strategies (e.g. use of time/temperature integrators) that are effective in ensuring the control of C. botulinum toxin formation.PathogensGrowth of Staphylococcus aureus is also a concern in fresh finfish in these packaging types, because the reduced oxygen environment may give it a competitive advantage over the normal spoilage organisms. The formation of a heat stable toxin by this pathogen should also be considered in your hazard analysis of fresh fish in reduced oxygen packaging.MetalAnd the last hazard is metal fragments. This hazard is usually significant if worn, damaged, or broken equipment could contribute metal fragments to the product - for example, mechanical pickers or filleters, wire-mesh belts, saws, or mixing, blending, chopping, dispensing or portioning equipment. It is usually not significant if you have evidence that a control for metal fragments will be provided by a subsequent processor.MODULE 2PERFORMING A HAZARD ANALYSISPROCESSOR OF COOKED, READY-TO-EAT CRUSTACEAN(INCLUDING CRAB, CRAYFISH, SHRIMP, LOBSTER)This module continues the discussion of performing a hazard analysis. It covers a different product - cooked, ready-to-eat crustaceans. These include, cooked crab, crayfish, shrimp, and lobster.Remember, there are some up front steps for the hazard analysis that were discussed in Module 1. They will not be covered again here. You can use the checklist that was also discussed in Module 1 as a quick reference.Specie Related HazardsSome of the hazards that were covered in the previous module, also apply to cooked, ready-to-eat crustaceans. As a result, the discussion for those hazards - in particular, ASP, PSP, and environmental contaminants (e.g. pesticides) - will be abbreviated here. The process you use to evaluate these hazards and to determine their significance in cooked crustaceans is the same as you used for finfish. The only difference is you use Table 3-2 (page 34), which covers invertebrate hazards.Aquaculture DrugsThe next hazard is aquaculture drugs. Again, there are some similarities to finfish, but there is enough of a difference to cover it separately here. Look in column 7 of Table 3-2 for those species that have a potential aquaculture drug hazard.Like finfish, the hazard is usually significant at the receiving step if you receive the fish directly from the grower - not if you receive it from another processor. Remember that there are some regions and some species in which aquaculture drugs are not used. If this is the case, there is no hazard.However, unlike finfish, aquaculture drugs are also usually significant after receiving when lobster are being held in a pound. That is because, in some holding pounds drugs are used to combat animal diseases that can occur in intense culture.。

单冻虾仁H A C C P计划书HC/02-2002目录前言关于建立HACCP办公室的通知关于实施2OO2年单冻虾仁HACCP计划的发布令第一部分:一般信息和产品描述第二部分:SSOP计划第三部分:工艺叙述和流程图第四部分:危害分析第五部分:HACCP计划表第六部分:GMP/SSOP/HACCP记录表(见单册本)第七部分:其它前提计划A、人员培训计划B、工厂维修保养计划C、产品召回计划D、产品识别代码计划E、冻虾仁产品纠偏计划F、HACCP计划验证程序附表:·美国FDA和EPA的指导标准参考文献:CAC CCFFP《国际虾类推荐标准(CAC/RCP17-1978)》前言本HACCP计划书设计于单冻虾仁产品的生产,单冻虾仁的原料来自于中国东海海域,鲜虾原料由当地渔船捕捞(渔船经官方机构注册),由本公司采购员在海上收购或码头直接收购。
冻虾原料来自于中国检验检疫局注册的加工冷冻厂,由本公司采购员采购。
本计划书按照“AFDO、HACCP培训教程”、“FDA水产和水产品危害控制指南”、“中国《出口食品生产企业卫生要求》”及“国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》”的要求加以制定,本计划书的目的是为了达到中国《出口食品生产企业卫生要求(GMP)》第五条的要求。
HACCP(危害分析和关键控制点)是一种食品安全预防控制体系,食品生产企业利用HACCP体系对影响产品的各种危害因子进行评估,通过对产品的危害因子分析,确定关键控制点,实施对危害因子的有效控制,使食品危害能防止或消除、或降低到可接收水平。
其目的是确保产品的安全性,并能用稳健方式生产的产品是安全的。
本公司建于1999年,由于中国已加入了WTO,中国的经济贸易融入了全球经济贸易一体化的格局;在这样的时况下,本公司为了符合全球经济贸易一体化的发展要求,已于2002年1-5月按照美国FDA21CFR第110部分——食品生产的现行良好操作规范的规定(CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING,PACKING,ORHOLDING HUMAN FOOD)及中国《出口食品生产企业卫生要求》的要求进行了改建,力争符合规范的规定。
生效日期2018年08月22日修订日期2018年08月20日HACCP计划书(速冻调制食品)生效日期2018年08月22日修订日期2018年08月20日生效日期2018年08月22日修订日期2018年08月20日目录一、食品安全(HACCP)小组成员介绍 (1)二、食品安全小组职责与权限 (2)三、产品的特性描述及预期用途说明 (3)四、流程图、过程步骤及控制措施 (5)五、危害分析及控制措施选择 (7)六、HACCP计划书 (14)七、附则 (17)八、附件 (17)生效日期2018年08月22日修订日期2018年08月20日一、食品安全(HACCP)小组成员介绍序号姓名部门职务学历/专业工作经验1林丽美品管部品管部经理本科/食品工程从事食品质量管理20年2李志强研发部研发部总监本科/食品工程从事食品研发20年3陈蕾品管部品管主任大专/食品工程从事食品检验9年4纪玉兰品管部QA大专/生物技术从事食品行业7年5张万珠销售中心客服主任大专/中文从事售后服务7年6乐明香采购部采购部经理大专/国际经济与贸易从事物控管理15年7任庆存生产部厂长本科/食品工程从事食品生产管理20年8陈宜衍研发部项目经理大专/生物技术从事食品研发管理15年9卢志敏生产部生产经理大专/生物制药从事食品生产管理7年10陆泽莉生产计划部生产计划主任大专/国际贸易从事食品行业7年生效日期2018年08月22日修订日期2018年08月20日二、食品安全小组职责与权限(一)食品安全小组(1)制订HACCP计划。
(2)修改、验证HACCP计划。
(3)监督、实施HACCP计划。
(4)编写PRP和OPRP文件。
(5)负责对全体员工进行培训。
(6)应具有较强的责任心和认真、实事求是的工作态度。
(二)食品安全小组组长(1)确保按照体系要求建立、实施、保持和持续改进食品安全管理体系。
(2)定期向总经理报告食品安全管理体系的运行情况以及有效性和适宜性,以供评审和作为食品安全管理体系改进的基础。
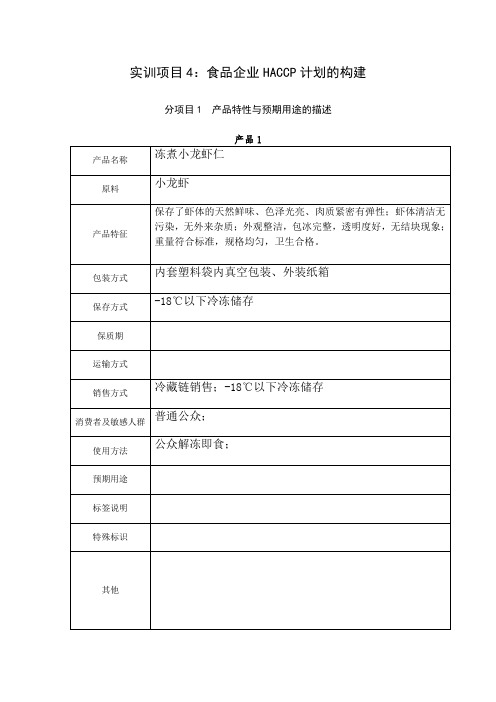
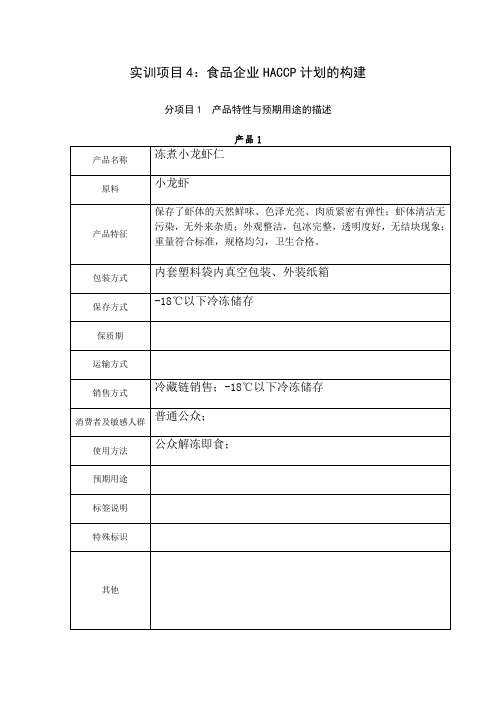
实训项目4:食品企业HACCP计划的构建分项目1 产品特性与预期用途的描述产品1产品名称冻煮小龙虾仁原料小龙虾产品特征保存了虾体的天然鲜味、色泽光亮、肉质紧密有弹性;虾体清洁无污染,无外来杂质;外观整洁,包冰完整,透明度好,无结块现象;重量符合标准,规格均匀,卫生合格。
包装方式内套塑料袋内真空包装、外装纸箱保存方式-18℃以下冷冻储存保质期运输方式销售方式冷藏链销售;-18℃以下冷冻储存消费者及敏感人群普通公众;使用方法公众解冻即食;预期用途标签说明特殊标识其他分项目2 冻煮小龙虾仁生产加工工艺流程图的编制、确认和控制1、冻煮小龙虾仁生产工艺流程图原料虾验收↓原料虾挑选↓运输清洗↑↓冷藏蒸煮↑↓装箱冷却↑↓冻结包装材料去头、去壳、去黄、↑↓去肠、分级真空装袋验收↓↑↓半成品验收→称重、装袋←储存2、冻煮小龙虾仁生产工艺描述原料虾验收:原料的好坏直接影响到成品质量的好坏,因此,原料验收与挑选是很重要的一环。
供应商必须提供产地证明及标签;经检验合格后验收。
验收原料时首先要观察整批虾体的清洁程度并嗅其气味,一般在较为清洁卫生的环境中生长的小龙虾,虾体色泽鲜艳、洁净,嗅之无异味。
反之,生长在农田、死水沟里的龙虾,其虾体表面污秽不洁,且很难洗净,嗅之有污泥味,甚至有农药或其它异味,此种虾应拒绝收购。
严格挑出死虾及老壳虾。
原料虾挑选:严格挑出死虾、病虾和老壳虾,以及小虾和不符合规格大小的虾。
原料虾清洗:用清水喷淋清洗3分钟,水温不高于10℃并且,个别不易清洗的应用刷子进行刷洗。
蒸煮:蒸煮在100℃开水中进行,蒸煮时间的长短应视不同季节、虾壳的不同厚度、虾体的大小等来决定。
蒸煮时间过短,会造成杀菌不彻底;而蒸煮时间过长,会造成出品率降低,虾仁弹性及口感变差。
冷却:自来水预冷及冰水冷却的目的是使蒸煮的龙虾立即降温,以达到保证虾仁品质在加工过程中不发生改变的目的。
预冷冷却用水应在使用前化验检测,若在微生物指标上未达到生活饮用水的标准,可加适量的含氯消毒液(如二氯异氰尿酸钠、三氯异氰尿酸钠等)使其达到标准,并在使用过程中及时更换,保证预冷冷却用水不对虾体造成新的污染。
单冻虾仁H A C C P计划书HC/02-20022002-4-10 发布 2002-5-15实施名称: 水产有限公司法人代表:地址:电话:0086-0 邮编:316101 传真:0086- 0本计划书由质技部编印目录前言关于建立HACCP办公室的通知关于实施2OO2年单冻虾仁HACCP计划的发布令第一部分:一般信息和产品描述第二部分:SSOP计划第三部分:工艺叙述和流程图第四部分:危害分析第五部分:HACCP计划表第六部分:GMP/SSOP/HACCP记录表(见单册本)第七部分:其它前提计划A、人员培训计划B、工厂维修保养计划C、产品召回计划D、产品识别代码计划E、冻虾仁产品纠偏计划F、HACCP计划验证程序附表:·美国FDA和EPA的指导标准参考文献:CAC CCFFP《国际虾类推荐标准(CAC/RCP17-1978)》前言本HACCP计划书设计于单冻虾仁产品的生产,单冻虾仁的原料来自于中国东海海域,鲜虾原料由当地渔船捕捞(渔船经官方机构注册),由本公司采购员在海上收购或码头直接收购。
冻虾原料来自于中国检验检疫局注册的加工冷冻厂,由本公司采购员采购。
本计划书按照“AFDO、HACCP培训教程”、“FDA水产和水产品危害控制指南”、“中国《出口食品生产企业卫生要求》”及“国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》”的要求加以制定,本计划书的目的是为了达到中国《出口食品生产企业卫生要求(GMP)》第五条的要求。
HACCP(危害分析和关键控制点)是一种食品安全预防控制体系,食品生产企业利用HACCP体系对影响产品的各种危害因子进行评估,通过对产品的危害因子分析,确定关键控制点,实施对危害因子的有效控制,使食品危害能防止或消除、或降低到可接收水平。
其目的是确保产品的安全性,并能用稳健方式生产的产品是安全的。
本公司建于1999年,由于中国已加入了WTO,中国的经济贸易融入了全球经济贸易一体化的格局;在这样的时况下,本公司为了符合全球经济贸易一体化的发展要求,已于2002年1-5月按照美国FDA21CFR第110部分——食品生产的现行良好操作规范的规定(CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING,PACKING,ORHOLDING HUMAN FOOD)及中国《出口食品生产企业卫生要求》的要求进行了改建,力争符合规范的规定。
本公司具有操作人员50多人(不包括临时工),其中管理人员10人(包括经浙江出入境检验检疫局培训和发证的HACCP人员8人)。
公司建立了HACCP办公室负责对公司的GMP/SSOP/HACCP系统实施进行管理。
关于建立HACCP办公室的通知为了按照国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》的要求建立和实施HACCP体系组织对国外单冻虾仁产品的生产和出口,公司经研究决定建立HACCP办公室。
办公室的有关工作人员任命如下:HACCP办公室主任王军民,负责本公司HACCP计划的领导职责,包括协调HACCP/SSOP计划的制定、实施和监督,确保公司按照所制定的HACCP计划运作。
HACCP办公室的人员包括业经浙江出入境检验检验局培训并发证及公司内经培训的人员组成;包括(总经理)、(生产部经理)、(化验员)、(粗加工车间主任)、(精加工车间主任)、(精加工车间)、(氨机车间)、(质量体系内审员;聘请兼职)组成。
HACCP办公室负责本公司的SSOP/HACCP计划书的制定;评估和修改SSOP/HACCP计划书,并进行危害分析以及审查GMP/SSOP/HACCP 记录。
特此通知舟山市水产有限公司2002年5月15日关于实施2002年单冻虾仁HACCP计划的发布令为了保证本公司生产的单冻虾仁符合国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》和中国《出口食品生产企业卫生要求(GMP)》的要求,现将经HACCP办公室于2002年4月份制定的SSOP/HACCP计划书印发下达给你们,请你们严格按照计划书认真执行。
做到从原料到成品由专人负责,严格把关,不断健全和完善食品安全预防控制体系,确保产品在符合国际食品法典委员会规范及中国《出口食品生产企业卫生要求(GMP)》的要求基础上进行加工。
本颁布令从2002年5月16日起开始实施。
本公司各有关部门、各生产岗位具体操作员工均需毫无例外地执行此颁布令。
总经理(兼HACCP办公室主任):2002年5月15日第一部分:一般信息和产品描述A、一般资料企业名称:FIRM NAME:企业地址:ADDRESS:注册代号:****/*****HACCP计划首次批准时间:2000年5月15日B.产品描述产品种类:虾类(shrimp)最终产品单体结冻生虾仁(individually quick frozen raw,peeled shrimp/prawn;简称单冻虾仁)C.包装方式:内套塑料袋(1kg/袋),外装纸箱(12袋/箱或10袋/箱),另可根据客户要求进行调整。
D.销售和贮存方法:冷藏链销售;冷冻贮存和发运(-18℃以下)。
E.预期用途和消费者:购买后消费前充分加热后方可食用;消费对象为普通公众。
工艺叙述和流程图A、工艺叙述:公司名称:公司地址:产品:单冻生虾仁加工过程/步骤:1、鲜虾接收:公司采购员直接从渔民渔船上收购或在码头直接收购;渔民在海上有时使用亚硫酸盐防腐剂对鲜虾进行处理,以防止虾体黑变。
经检验合格后接收(应符合GB2741-94海虾卫生标准)。
原料虾运至公司原料接收区,在接收区清洗后,用4℃以下的冰水降温,然后用清洁的鱼盘(筐)层冰层虾做保鲜处理后,存放于预冷库。
2、片冰保鲜:原料虾接收后存放在清洁的鱼盘(筐)中,用片冰保鲜,层冰层虾片冰压顶。
预冷库的温度低于℃,虾体几何中心温度低于4℃。
若连续2小时库温超过℃,虾体温度高于4℃,该批原料虾必须在2小时内处理完成(整个生产流程不超过4小时,特殊情况不超过6小时;实验室应检测金黄色葡萄球菌)。
3、冻虾原料:直接从有国家质量监督检验检疫总局注册的加工冷冻厂收购,产品是否含有亚硫酸盐(以SO2 计),供应商必须提供证明及标签;经检验合格后验收。
4、解冻:将冻虾原料放在粗加工车间操作台上空气自然解冻或流动水解冻,室温不超过20℃,解冻至半化冻状态。
5、去头去壳:保鲜加冰的鲜虾从预冷库进入粗加工车间;分送到操作台上,用手工去头、去壳。
冻虾原料解冻后直接在操作台上进行。
然后用清水清洗后送到精加工车间进行分级。
粗加工车间环境温度控制在20℃以下。
6.清洗:虾仁清洗用水的水温尽量不高于10℃;清洗时不断搅动,洗去泥沙及杂质;清洗用水应及时更换,避免交叉污染。
虾仁清洗后用4℃以下冰水浸泡降温。
10.分级:剥好的虾仁清洗后应用冰水浸洗作降温处理,然后在一张不锈钢桌面上,人多虾少的情况下迅速分好规格。
分规格的同时应拣出鲜度差、断裂的虾及杂质;要求分选的规格比标定规格只数少几只,由班组长检测只数。
并存放在各自的塑料筛中。
每档规格放标记指示。
虾仁分级规格要求:只数/磅(LB);也可根据客户的要求进行调整。
8.泡液:根据客户的要求,将清洗降温分级后的虾仁立即放入盛有多聚磷酸盐(品质改良剂,符合GB2760-86食品添加剂使用卫生标准)溶液的容器中;水:虾仁:多聚磷酸盐的比例一般情况下分别为50:70:2%左右(KG), 溶液温度控制在℃以下,半成品几何中心温度控制在4℃以下,溶液浸泡时间根据客户的要求,但应小于4小时(根据进口国的法规要求)。
精加工车间环境温度控制在20℃以下。
9.挑选:根据泡液的效果及检验结果而定。
如果杂质或每磅只数不符合要求,就应把泡液后的虾仁进行挑选,符合规定要求后再进行清洗。
10、清洗:用清洁的流动水洗净后放在搁架上沥水3分钟后进行冻结。
11、单冻:将沥水后的虾仁摆放在单冻机上,加工人员将其均匀地放置于输送带上,随时目测挑选零星杂质。
用-30℃以下的冻结温度进行冻结。
12、半成品冻藏:将分级单冻后的不同规格虾仁装袋(其容量为10Kg),放入周转箱,置于-18℃以下的半成品冻藏库中按规格、生产日期或批号堆放使用。
13、镀冰衣:从冻藏库出库的单冻虾仁镀冰衣时水温控制在0-4℃,水质符合饮用水标准。
浸水时间在3-5秒之间;镀冰衣后虾仁表面光滑美观,防止干耗。
镀冰衣量按照客户要求。
镀冰衣后的虾仁粘连须分开。
14、称量:将镀冰衣后的单冻虾仁使用鉴定准确的衡器按以合同规定的产品净重和镀冰衣产品解冻后的净重为依据进行称量。
15、包装材料接收:用清洁、密封和保养良好的车辆运输,经HACCP办公室会同车间检验合格后,指定批号分别存放于干燥的物料仓库内。
16、包装材料贮藏:包装材料按内包装和外包装材料分别存放在物料包装仓库内,加盖塑料薄膜以防止包装材料受到污染。
17、包装/贴标:把镀冰衣后的虾仁(或用不锈钢漏斗)装入清洁无毒卫生的塑料袋中,封口。
如产品含有亚硫酸盐在10—100PPM 之间的(以SO2计;根据进口国的法规要求和客户的要求),必须贴标签声明。
18、金属检测:把封口后的产品放在金属探测仪的输送带上进行金属探测,经探测合格的产品才能装外纸箱。
若发现有金属异物,立即捡出,单独隔离放置,查明原因并记录。
19、装外纸箱:产品经金属探测后,按客户要求装入外纸箱内并封箱;外纸箱上标明产品生产的日期、企业代码和批号。
包装完毕,成品送入成品冻藏库。
21、冻藏:所有成品立即送入-18℃以下冻藏库中,按规格、批号分别推垛。
22、出厂发运:所有货运冷冻集装箱装运前应检查车箱内是否清洁卫生。
箱内温度预冷至10℃以下放可装货;装货完毕,箱内温度制冷至-18℃以下放可起运。
B、单冻生虾仁工艺流程图2.9.?? ??? ?18.???注:泡药工序根据客户要求。
SSOP计划书卫生标准操作程序(2002年第一版)企业名称:企业地址:关于实施2002年SSOP计划的发布令2002年5月份,公司HACCP办公室根据中国《出口食品生产企业卫生要求(GMP)》、国际食品法典委员会《危害分析和关键控制点(HACCP)体系及其应用准则》的要求,并参考了美国FDA21CFR 第110部分---食品生产的现行良好操作规范的规定,制定了第一版SSOP计划书,请你们严格按照本计划书认真执行。
做到从原料到成品由专人负责,严格把关,不断健全和完善食品安全预防控制体系(主要指卫生管理体系),确保产品在符合国际食品法典委员会规范及中国《出口食品生产企业卫生要求(GMP)》的要求基础上进行加工。
本颁布令从2002年5月16日起开始实施。
本公司各有关部门、各生产岗位管理人员和具体操作员工均需毫无例外地执行此颁布令。