环己基异氰酸酯安全技术说明书
- 格式:docx
- 大小:33.29 KB
- 文档页数:5

安全技术说明书根据GB/T 16483-2008第 1 页 共 9 页LOCTITE PC 5070 TAPE 又名 Pipe Repair Kit TAPE安全技术说明书编号 : 157264V 001.4修订: 31.07.2012 发布日期: 05.07.2018化学品中文名称: LOCTITE PC 5070 TAPE 又名 Pipe Repair Kit TAPE企业信息:汉高(中国)投资有限公司 张衡路928号 201203 中国上海市浦东新区中国电话: +86-21-2891 8000 传真:+86-21-2891 5137生效日期: 31.07.2012应急信息: 应急电话:+86 532 8388 9090 (24小时)。
物质或混合物的分类根据GB 13690-2009 (化学品分类和危险性公示通则):危险分类 危险类别 接触途径 靶器官 急性毒性第4类 食入 严重眼损伤/眼刺激第2A 类 眼睛接触 特定目标器官毒性-单次接触 第3类肺 皮肤腐蚀/刺激 第2类 皮肤接触 致癌性 第2类 呼吸过敏性 第1A 类 吸入 皮肤敏化作用第1A类皮肤接触标签要素根据GB 15258-2009 (化学品安全标签编写规定):象形图:信号词:危险危险性说明:H302 吞咽有害。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
H315 对皮肤有刺激。
H351 怀疑会致癌。
H334 吸入可能导致过敏或哮喘病症状或呼吸困难。
H317 可能引起皮肤过敏。
防范说明(预防):P264处理后要彻底洗净P270使用本产品时不得进食、饮水或吸烟。
P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
P261避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
P271只能在室外或通风良好之处使用。
P201在使用前获取特别指示。
P202在读懂所有安全防范措施之前切勿搬动。
P281使用所需的个人防护设备。
P285如通风不足,须戴呼吸防护装置。

Substance Identifier task started on Sat Aug 6, 2011 at 2:44 PMExplored by Substance Identifier in REGISTRY.REGISTRY Answers1 for 3173-53-3_RN:3173-53-3_Explored Reactions containing Registry Number 3173-53-3_RN:3173-53-3_ as a product in CASREACT105 Reactions.Copyrights:CAPLUS: Copyright © 2009 American Chemical Society. All Rights Reserved. (The UK patent material in this product/service is UK Crown copyright and is made available withpermission. © Crown Copyright. The French (FR) patent material in this product/service is made available from Institut National de la Propriete Industrielle (INPI).)MEDLINE: Produced by the U.S. National Library of MedicineREGISTRY: Copyright © 2009 American Chemical Society. All Rights Reserved. (Some records contain information from GenBank(R). See also: Benson D.A., Karsch-Mizrachi I., LipmanD.J., Ostell J., Rapp B.A., Wheeler D.L. Genbank. Nucl. Acids Res. 28(1):15-18 (2000).Property values tagged with IC are from the ZIC/VINITI data file provided by InfoChem.) CAS Registry is a service mark of the American Chemical Society.CASREACT: Copyright © 2009 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided byInfoChem; INPI data prior to 1986; Biotransformations database compiled under thedirection of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.CHEMLIST, CHEMCATS: Copyright © 2009 American Chemical Society. All Rights Reserved.++NOT E: pr od uct (s ) dep en d o nre act io n c on di tio ns , Re ac tan ts : 2, Ca tal ys ts: 1, Solve nt s: 1,St ep s: 1, S tag es : 1Tetrahedron Letters, 51(48), 6301-6304; 2010 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+(MeO )2CO++C 6H 11NM e 2++NOT E: hi gh pr es su re, a uto cl av e u se d, Re ac tan ts : 2, Ca tal ys ts : 2, St ep s: 1, S tag es : 1Faming Zhuanli Shenqing Gongkai Shuomingshu, 101759602, 30 Jun 2010 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NOT E: 1:2:2:2 R O-THP:n-Bu4N[OCN]:P h3P:D DQ (m ol ar ra ti o), 100% c on ver si on,Re ac tan ts: 2, Re age nt s: 2, S olv en ts: 1,St ep s: 1, S tag es: 1∙Phosphorus, Sulfur and Silicon and the Related Elements, 184(10), 2525-2529; 2009 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)MeS+NOT E: 1) i ndu st ri al, 2) i nd us tri al,Re ac tan ts: 2, Re age nt s: 2, S olv en ts: 2,St ep s: 2, S tag es: 4, Most st age s inan y one s te p: 3Ital. Appl., 2005MI1284, 07 Oct 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NCONOT E: in du str ia l,Re ac tan ts: 1, Re age nt s: 2, S olv en ts: 1,St ep s: 1, S tag es: 3Ital. Appl., 2005MI1284, 07 Oct 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)(Cl 3CO )2C =O+NH 2NOT E: Re ac tan ts : 2, Re agent s: 1, Solv en ts : 1,St ep s: 1, S tag es : 1Hecheng Huaxue, 16(5), 591-592; 2008 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)MeOCN+N H 2N CONOT E: re ac tan t as sum ed ,Re ac tan ts : 2,St ep s: 1, S tag es : 1Russian Journal of Organic Chemistry, 44(12), 1765-1772; 2008 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)ClC O 2C Cl 3+NOT E: Re ac tan ts : 2, Re age nt s: 1, S olv en ts : 1,St ep s: 1, S tag es : 1Faming Zhuanli Shenqing Gongkai Shuomingshu, 101284810, 15 Oct 2008 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+M eSNOT E: 1) s afe ty , 2) sa fet y,Re ac tan ts : 2, Re age nt s: 2, S olv en ts : 2,St ep s: 2, S tag es : 3, Mo st st age s in an y one s te p: 2Synthesis, (10), 1612-1618; 2008 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NOT E: sa fe ty,Re ac tan ts : 1, Re age nt s: 2, S olv en ts : 1, St ep s: 1, S tag es : 2Synthesis, (10), 1612-1618; 2008 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)CO 2+N HNOT E: Na H, Gl ym e, Am in ati on , C-A mi nat io n, Co nd ens at io n, Re ac tan ts : 2, Re age nt s: 1, S olv en ts : 1, St ep s: 1, S tag es : 1Organic Reactions (Hoboken, NJ, United States), 25, No pp. given; 1977 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NOT E: ch em ose le ctive , opt im iz ati on st ud y,Reac tan ts : 2, Ca tal ys ts : 1, Sol ve nt s: 1, St eps: 1, S tages : 1∙Turkish Journal of Chemistry, 31(1), 35-43; 2007 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NOT E: ch em ose le ct ive , opt im iz ati on st ud y,Re ac tan ts : 2, Ca tal ys ts : 1, Sol ve nt s: 1, St ep s: 1, S tag es : 1∙Turkish Journal of Chemistry, 31(1), 35-43; 2007 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)0=C (NH 2)2+N H 2NOT E: 2) t her ma l,Re actan ts :2, So lvent s: 1, St ep s: 2, S tag es : 2Faming Zhuanli Shenqing Gongkai Shuomingshu, 1966491, 23 May 2007 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)42%NOT E: th ermal ,Re ac tan ts : 1,St ep s: 1, S tag es : 1Faming Zhuanli Shenqing Gongkai Shuomingshu, 1966491, 23 May 2007 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)Me 3SiC l++O=C Cl 2(St ep 2.1)NOT E: Re ac tan ts : 3, Re age nt s: 1, S olv en ts : 1, St ep s: 2, S tag es : 2Russian Journal of General Chemistry, 76(3), 469-477; 2006 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+C O 2+(Me 3S i)2NH+NOT E: 2) t her mal,Re ac tants : 3, Re age nt s: 1, S olv ents : 1,Step s: 2, S tag es : 2Russian Journal of General Chemistry, 76(3), 469-477; 2006 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N H+55%NOT E: th ermal ,Re ac tants : 1, Reage nt s: 1, St ep s: 1, S tag es : 1Russian Journal of General Chemistry, 76(3), 469-477; 2006 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O=C Cl 2+55%NOT E: Re ac tan ts : 2, So lve nt s: 1, St ep s: 1, S tag es : 1Russian Journal of General Chemistry, 76(3), 469-477; 2006 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)di mer s an d t ri mer s99%NOT E: tr im eris f orm ed ,Re ac tan ts : 1, Ca talys ts : 1, Sol ve nt s: 1,St ep s: 1, S tag es : 1PCT Int. Appl., 2005113626, 01 Dec 2005 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONOT E: di me r = 4% yie ld , t ri me r = 11% yi el d,Re ac tan ts : 1, Ca tal ys ts : 1, Sol ve nt s: 1, St ep s: 1, S tag es : 1PCT Int. Appl., 2005113626, 01 Dec 2005 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONC Odi mer s an d t ri mer s NOT E: di me r =62% yi el d, tr im er = 2% yi el d,Re actan ts: 1, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONC Odi mer s an d t ri mer s64%NOT E: on ly di me r is fo rme d,Re actan ts: 1, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONC Odi mer s an d t ri mer s58%NOT E: on ly di me r is fo rme d,Re ac tan ts: 1, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONOT E: on ly trim er is f orm ed,Re ac tan ts: 1,Ca talys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONC Odi mer s an d t ri mer s14%NOT E: on ly di me r is fo rme d,Re ac tan ts: 1, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CONC Odi mer s an d t ri mer s NOT E: di me r = 55%yi el d, tr im er = 18% y ie ld,Re ac tan ts: 1, Ca tal ys ts: 1, Sol ve nt s:1,Step s: 1, S tag es: 1PCT Int. Appl., 2005113626, 01 Dec 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+C O2NCO NOT E:Re ac tan ts: 2, Re age nt s: 2, S olv en ts: 1,St eps: 1, S tag es: 2Faming Zhuanli Shenqing Gongkai Shuomingshu, 1590369, 09 Mar 2005CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NOT E: me th od is s ele ct ive f or co nv ers io n of pr ima ry a lco ho ls in p res en ce ofse co nda ry a nd te rti ar y alc oh ol, t hi ols, and t ri met hy lsi ly l eth er s;al te rna te p rep n. de sc ri bed,Re ac tan ts: 2, Re age nt s: 2, S olv en ts: 1,St ep s: 1, S tag es: 1∙Synthesis, (12), 1955-1958; 2005 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NOT E: me th od is s ele ct ive f or co nv ers io n ofpr ima ry a lco ho ls in p res en ce of se co nda rya nd te rti ary alc oh ol, t hi ols , and t ri met hy lsi ly l eth er s; al te rna te p rep n. de sc ri bed ,Reac tan ts : 2, Re age nt s: 2, S olv en ts : 1, St eps: 1, S tag es : 1∙Synthesis, (12), 1955-1958; 2005 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)(Cl 3CO )2C =O+NOT E: Re ac tan ts : 2, So lve nt s: 1, St ep s: 1, S tag es : 2Zhongguo Yaowu Huaxue Zazhi, 13(3), 170-171; 2003 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)ClC O 2C Cl 3+NOT E: Re ac tan ts : 2, So lve nt s: 1, St ep s: 1, S tag es : 1Faming Zhuanli Shenqing Gongkai Shuomingshu, 1436772, 20 Aug 2003CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N H2+(Cl3CO)2C=ONOT E: ei th er so lv ent,Re ac tants: 2, So lve nt s: 2,St ep s: 1, S tag es: 1Huaxue Shiji, 24(6), 375; 2002CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O=C Cl2+NOT E: di me thy ls ul fox id e g av e sim il aryi el d,Re ac tan ts: 2, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1,S tag es: 2Ger. Offen., 10152119, 30 Apr 2003CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O=C Cl2+NOT E: Re ac tan ts: 2, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 2Ger. Offen., 10152118, 30 Apr 2003CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O=C Cl 2+NOT E: ca ta lys t Cy anex 923 g av e s im ila r yi eld ,Re ac tan ts : 2,Ca tal ysts : 1, Sol ve nt s: 1,St ep s: 1, S tag es : 2Ger. Offen., 10152117, 30 Apr 2003 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+C O +NOT E: El ec tro ch em., di alk yl ur ea:is ocy an at e=92:8, Re ac tan ts : 2, Ca tal ys ts : 2, Sol ve nt s: 1, St ep s: 1, S tag es : 1Journal of Molecular Catalysis A: Chemical, 176(1-2), 73-78; 2001 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O MeNOT E: gr ee n c he m., opt imi za ti on,Re ac tan ts:1, Ca tal ys ts: 1, Sol vent s: 1,St ep s: 1, S tag es: 1Tetrahedron Letters, 43(9), 1673-1676; 2002CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)OCN+F93% NOT E: Re ac tan ts: 1, Re age nt s: 1, C ata ly st s: 1, So lven ts: 1,St ep s: 1, S tag es: 1Zhongguo Yaowu Huaxue Zazhi, 11(1), 49-50; 2001CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)O=C Cl2+NOT E: Re ac tan ts: 2, Ca tal ys ts: 1, Sol ve nt s: 1,St ep s: 1, S tag es: 2Ger. Offen., 19942299, 08 Mar 2001CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)CO 2+NOT E: Re ac tan ts :2, Re age nt s: 2, S olv en ts : 1, Step s: 1, Stag es : 3Journal of Organic Chemistry, 64(11), 3940-3946; 1999 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the directionof Professor Dr. Klaus Kieslich.)CO 2++i -P rO80%NOT E: Re ac tan ts :2, Reage nt s: 1, S olv en ts : 1, Step s:1, S tag es : 1Tetrahedron Letters, 40(2), 363-366; 1999 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)N CO98%NOT E: Re ac tan ts : 1, Ca tal ys ts : 2, Sol ve nt s: 1, St ep s: 1, S tag es : 1Can. Pat. Appl., 2141643, 10 Aug 1995CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NOT E: Re ac tan ts : 1, Re agent s: 1, C ata ly st s: 2, So lven ts: 2, Step s: 1, Stag es : 1Journal of Organic Chemistry, 60(17), 5430-3; 1995 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)+NCONOT E: Reac tan ts : 1, Re age nt s: 2, S olven ts: 1, St ep s: 1, S tag es : 1Journal of Organic Chemistry, 60(1), 257-8; 1995 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NC O90%NOT E: NA OH /BR 2, S OLU TI ON,Re ac tan ts : 1, Re age nt s: 1, S olv en ts : 1, St ep s: 1, S tag es : 1Journal of Chemical Technology and Biotechnology, 59(3), 271-7; 1994 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)Et 3N+NOT E: CA RB ON DI OX IDE , Reac tan ts : 2,St ep s: 1, S tag es : 1Journal of the Chemical Society, Chemical Communications, (8), 957-8; 1994 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NH 2NOT E: Re ac tan ts : 1, Reage nts: 1, C ata ly sts: 2, Solv en ts: 2, St ep s: 1, S tag es : 1Journal of the Chemical Society, Chemical Communications, (6), 679; 1994 CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided byInfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)82%NOT E: th er mal ,Re ac tan ts : 1, Ca tal ys ts : 1, St ep s: 1, S tag es : 1Jpn. Kokai Tokkyo Koho, 05186415, 27 Jul 1993CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)NOT E: th er mal,Re ac tan ts: 1, Ca tal ys ts: 1,St ep s: 1, S tag es: 1Jpn. Kokai Tokkyo Koho, 05186414, 27 Jul 1993CASREACT(Copyright (C) 2011 ACS. In addition to reactions indexed by CAS, CASREACT contains reactions derived from the following sources: ZIC/VINITI database (1974-1999) provided by InfoChem, INPI data prior to 1986, and Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich.)。
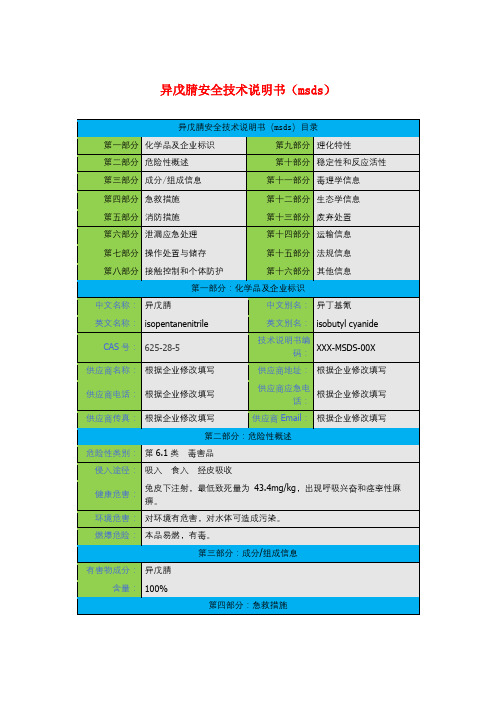

化学品安全技术说明书产品名称: 异氰酸酯按照GB/T 16483、GB/T 17519 编制修订日期: 最初编制日期:版本:第1部分化学品及企业标识化学品中文名:异氰酸酯化学品英文名:isocyanic acid企业名称:企业地址:传真:联系电话:企业应急电话:产品推荐及限制用途:For industry use only.。
第2部分危险性概述紧急情况概述:吞咽有害。
皮肤接触有害。
造成严重皮肤灼伤和眼损伤。
吸入有害。
吸入可能导致过敏或哮喘病症状或呼吸困难。
可引起呼吸道刺激。
GHS危险性类别:急性经口毒性类别 4急性经皮肤毒性类别 4皮肤腐蚀/ 刺激类别1B急性吸入毒性类别 4呼吸道致敏物类别 1特异性靶器官毒性一次接触类别 3标签要素:象形图:警示词:危险危险性说明:H302 吞咽有害。
H312 皮肤接触有害。
H314 造成严重皮肤灼伤和眼损伤。
H332 吸入有害。
H334 吸入可能导致过敏或哮喘病症状或呼吸困难。
H335 可引起呼吸道刺激。
防范说明:•预防措施:•P264 作业后彻底清洗。
•P270 使用本产品时不要进食、饮水或吸烟。
•P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
•P260 不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
•P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
•P271 只能在室外或通风良好处使用。
•P284 [在通风不足的情况下] 戴呼吸防护装置•事故响应:•P301+P312 如误吞咽:如感觉不适,呼叫解毒中心/ 医生•P330 漱口。
•P302+P352 如皮肤沾染:用水充分清洗。
•P312 如感觉不适,呼叫解毒中心/医生•P321 具体治疗 ( 见本标签上的…… )。
•P362+P364 脱掉沾染的衣服,清洗后方可重新使用•P301+P330+P331 如误吞咽:漱口。
不要诱导呕吐。
•P303+P361+P353 如皮肤(或头发)沾染:立即脱掉所有沾染的衣服。

安全技术说明书页: 1/13 上海巴斯夫聚氨酯有限公司中国安全技术说明书日期 / 修订: 16.07.2007版本: 4.0产品: LUPRANATE T-80 Ex Shanghai(30462599/SDS_GEN_CN/ZH)印刷日期 28.08.20091. 物质/制剂及公司识别LUPRANATE T-80 Ex Shanghai公司:上海巴斯夫聚氨酯有限公司中国 上海市上海化学工业区楚华路25号邮政编码 201507电话: +86 21 3750 3399-3000传真号: +86 21 6712-1118E-mail地址: liun@紧急情况资料:巴斯夫紧急热线中心(中国)电话: +86 21 5861-1199Company:Shanghai BASF Polyurethane Co.,Ltd.25 Chu Hua Road, Shanghai Chemical Industry Park(SCIP)Shanghai 201507, CHINA Telephone: +86 21 3750 3399-3000 Telefax number: +86 21 6712-1118E-mail address: liun@ Emergency information:Emergency Call Center (China): Telephone: +86 21 5861-11992. 成分/组分信息化学性质甲苯二异氰酸酯(TDI) (内容物 (W/W): 100%)上海巴斯夫聚氨酯有限公司中国安全技术说明书日期 / 修订: 16.07.2007版本: 4.0产品: LUPRANATE T-80 Ex Shanghai(30462599/SDS_GEN_CN/ZH)印刷日期 28.08.2009美国化学文摘号: 26471-62-5EC号: 247-722-4检索号: 615-006-00-43. 危险性识别吸入很毒.对眼睛,呼吸系统和皮肤有刺激性.吸入和皮肤接触可能会导致过敏.对水中生物体有害, 可能导致长期的对水环境的不利影响.有限证据表明有致癌影响.4. 急救措施一般建议:立即脱掉受污染的衣物。

化学品安全技术说明书公司地址:上海化学工业区奉贤分区银工路28号E栋楼客服热线:400-133-2688 1 化学品及企业标识1.1 产品标识符化学品俗名或商品名:氯乙基异氰酸酯CAS No.:1943-83-5别名:异氰酸-2-氯乙酯;2-氯异氰酸乙酯;1.2 鉴别的其他方法无数据资料1.3 有关的确定了的物质或混合物的用途和建议不适合的用途仅供科研用途,不作为药物、家庭备用药或其它用途。
2 危险性概述2.1 GHS分类无数据资料2.2 GHS 标记要素,包括预防性的陈述危害类型GHS02:易燃物; GHS07:感叹号; GHS08:健康危害;信号词 【危险】危险申明H226 易燃液体和蒸气。
H302 如果吞食有害健康。
H312 皮肤接触有害健康。
H315 引起皮肤过敏。
H319 造成了严重的眼睛发炎。
H332 吸入有害健康。
H334 吸入可能引起过敏或哮喘症状或呼吸困难。
H335 可能引起呼吸道发炎。
警告申明P261 避免吸入粉尘/烟/气体/烟雾/蒸汽/喷雾。
P280 戴防护手套/防护服/护眼/防护面具。
P305+P351+P338 如进入眼睛:用水小心清洗几分钟。
如果可以做到,摘掉隐形眼镜,继续冲洗。
P342+P311 如果出现呼吸道症状:呼叫解毒中心/医生。
RSHazard symbol(s) XnR-phrase(s) R34;R42;R25;RS-phrase(s) S26;S362.3 其它危害物-无3 成分/组成信息3.1 物质分子式 - C3H4ClNO分子量 - 105.524 急救措施4.1 必要的急救措施描述一般的建议请教医生。
向到现场的医生出示此安全技术说明书。
如果吸入用大量水彻底冲洗至少15分钟并请教医生。
在皮肤接触的情况下用肥皂和大量的水冲洗。
立即将患者送往医院。
请教医生。
在眼睛接触的情况下无数据资料如果误服用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
4.2 最重要的症状和影响,急性的和滞后的主要症状和影响,急性和迟发效应咳嗽,呼吸短促,头痛,恶心,呕吐4.3 及时的医疗处理和所需的特殊处理的说明和指示如必要的话,戴自给式呼吸器去救火。

第一部分化学品及企业标识化学品中文名:异氰酸环己酯化学品英文名:cyclohexyl isocyanate;isocyanatohexane化学品别名:环己基异氰酸酯CAS No.:3173-53-3EC No.:221-639-3分子式:C7H11NO第二部分危险性概述| 紧急情况概述液体。
易燃,其蒸气与空气混合,能形成爆炸性混合物。
有严重损害眼睛的危险。
吸入有剧毒。
| GHS 危险性类别根据GB 30000-2013化学品分类和标签规范系列标准(参阅第十六部分),该产品分类如下:易燃液体,类别3;皮肤腐蚀/刺激,类别1;眼损伤/眼刺激,类别1;急毒性-吸入,类别2。
| 标签要素象形图警示词:危险危险信息:易燃液体和蒸气,造成严重皮肤灼伤和眼损伤,造成严重眼损伤,吸入致命。
预防措施:远离热源、热表面、火花、明火以及其它点火源。
禁止吸烟。
保持容器密闭。
容器和接收设备接地和等势联接。
使用不产生火花的工具。
采取措施,防止静电放电。
不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
作业后彻底清洗。
只能在室外或通风良好之处使用。
戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
[在通风不足的情况下]戴呼吸防护装置。
事故响应:立即呼叫中毒急救中心/医生。
沾染的衣服清洗后方可重新使用。
如误吸入:将受人转移到空气新鲜处,保持呼吸舒适的体位。
如误吞咽:漱口。
不要诱导呕吐。
如皮肤(或头发)沾染:立即去除/脱掉所有沾染的衣服。
用水清洗皮肤或淋浴。
如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
安全储存:存放处须加锁。
存放在通风良好的地方。
保持容器密闭。
存放在通风良好的地方。
保持低温。
废弃处置:按照地方/区域/国家/国际规章处置内装物/容器。
物理化学危险易燃液体,其蒸气与空气混合,能形成爆炸性混合物。
健康危害在正常生产处理过程中,吸入本品的蒸气或气溶胶(雾、烟)可产生严重毒害作用,甚至可致命。
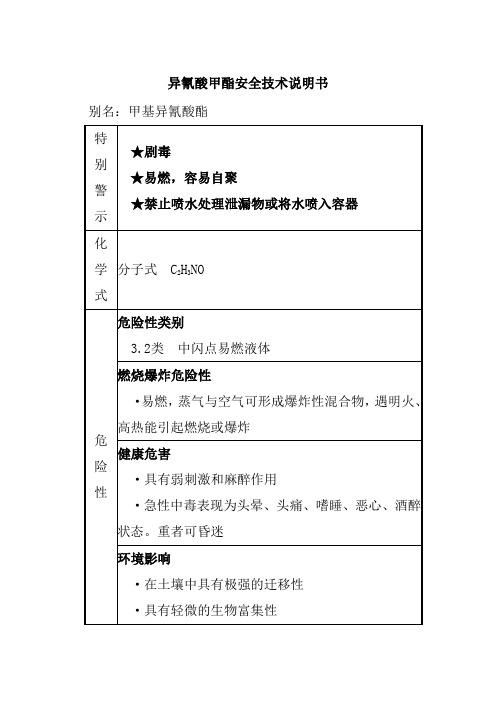

物质安全技术说明书(甲苯二异氰酸酯MSDS)1.化学品简介1.1中文名称甲苯-2,4-二异氰酸酯1.2英文名称toluene-2,4-diisocyanate1.3中文同义词甲苯-2,4-二异氰酸酯(TDI) 甲代亚苯基二异酸甲苯酯2,4-二异氰酸基甲苯2,4-甲苯二异氰酸酯1.4英文同义词2,4-tolylene diisocyanate 1-METHYL-1,3-PHENYLENE DIISOCYANATE2,4-DIISOCYANATOTOLUENE 2,4-TDI1.5CAS No. 584-84-91.6分子式C9H6N2O21.7分子量174.161.8 物理化学性质性状无色透明或淡黄色易燃液体。
有强烈的刺激气味。
熔点19.5~21.5℃沸点247℃相对密度1.217闪点127℃溶解性与乙醇(分解)、二甘醇、乙醚、丙酮、四氯化碳、苯、氯苯、煤油、橄榄油混溶。
2.危险性概述本品可燃,有毒,具刺激性,具致敏性。
3.急救措施3.1 皮肤接触脱去污染的衣着,用大量流动清水冲洗。
3.2 眼睛接触立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
3.3 吸入迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
3.4 食入用水漱口,给饮牛奶或蛋清。
就医。
4.消防措施4.1 危险特性遇明火、高热可燃。
与氧化剂可发生反应。
与胺类、醇、碱类和温水反应剧烈,能引起燃烧或爆炸。
加热或燃烧时可分解生成有毒气体。
其蒸气比空气重,能在较低处扩散到相当远的地方,遇火源会着火回燃。
若遇高热,容器内压增大,有开裂和爆炸的危险。
4.2 有害燃烧产物一氧化碳、二氧化碳、氧化氮、氰化氢。
4.3 灭火方法消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。


安全技术说明书TOLONATE HDB 75 MX第一部分 化学品及企业标识产品名称:TOLONATE HDB 75 MX名称:罗地亚(上海)国际贸易有限公司供应商:经销商:地址:上海市黄陂北路227号501室电话:+86 -21- 6375 8278传真:+86 -21- 6375 8040页: 1 / 9撤销并代替版本:日期 : 27/12/2006用途:油漆和清漆制造。
(更详细信息,请参见产品技术说明书)。
版本: 1第二部分 成分/组成信息>>制剂化学性质脂肪族聚异氰酸酯溶液。
:二异氰酸环己酯低聚物 (CAS 号:28182-81-2):~ 75 % - 自分类:Xi; R43 - EC 号: 500-060-2二甲苯 (CAS 号:1330-20-7):~ 12.5 % - EC 类别:R10 - Xn;R20/21 - Xi; R38 - (25 R)§ - EC 号: 215-535-7提供危险性的组分:乙酸甲氧基丙酯 (CAS 号:108-65-6):~ 12.5 % - EC 类别:R10- R36 - (19 N)§ - EC 号: 203-603-9二异氰酸环己酯 (杂质) (CAS 号:822-06-0):< 0.3 % - EC 类别:T; R23 - Xi; R36/37/38- R42/43 - (19 N)§ - EC 号:212-485-8具有危险的组分:二甲苯含有 ~ 20 % 乙苯(CAS 号:100-41-4)§:(N):指令67/548/EEC 附录I 中适应技术进步的新条目§:(R):指令67/548/EEC 附录I 中适应技术进步的修订条目>>更详细信息:第三部分 危险性概述最重要危险对人体有害对水生生物有害。
环境影响:吸入和皮肤接触对身体有害。
轻微刺激眼睛。
皮肤接触可致敏。



第1部分化学品及企业标识化学品中文名:环己基异氰酸酯化学品英文名:Cyclohexyl isocyanateCAS号:3173-53-3分子式:C7H11NO分子量:125.17产品推荐及限制用途:工业及科研用途。
第2部分危险性概述紧急情况概述:易燃液体和蒸气。
吞咽会中毒。
皮肤接触会中毒。
造成皮肤刺激。
造成严重眼刺激。
吸入致命。
吸入可能导致过敏或哮喘病症状或呼吸困难。
可引起呼吸道刺激。
GHS危险性类别:易燃液体类别3急性经口毒性类别3急性经皮肤毒性类别3皮肤腐蚀/刺激类别2严重眼损伤/眼刺激类别2急性吸入毒性类别2呼吸道致敏物类别1特异性靶器官毒性一次接触类别3标签要素:象形图:警示词:危险危险性说明:H226易燃液体和蒸气H301吞咽会中毒H311皮肤接触会中毒H315造成皮肤刺激H319造成严重眼刺激H330吸入致命H334吸入可能导致过敏或哮喘病症状或呼吸困难H335可引起呼吸道刺激防范说明:•预防措施:——P210远离热源/火花/明火/热表面。
禁止吸烟。
——P233保持容器密闭。
——P240容器和装载设备接地/等势联接。
——P241使用防爆的电气/通风/照明/设备。
——P242只能使用不产生火花的工具。
——P243采取防止静电放电的措施。
——P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
——P264作业后彻底清洗。
——P270使用本产品时不要进食、饮水或吸烟。
——P260不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
——P271只能在室外或通风良好处使用。
——P284[在通风不足的情况下]戴呼吸防护装置——P261避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
•事故响应:——P303+P361+P353如皮肤沾染:立即脱掉所有沾染的衣服。
用水清洗皮肤/淋浴。
——P370+P378火灾时:使用灭火器灭火。
——P301+P310如误吞咽:立即呼叫解毒中心/医生——P330漱口。
——P302+P352如皮肤沾染:用水充分清洗。
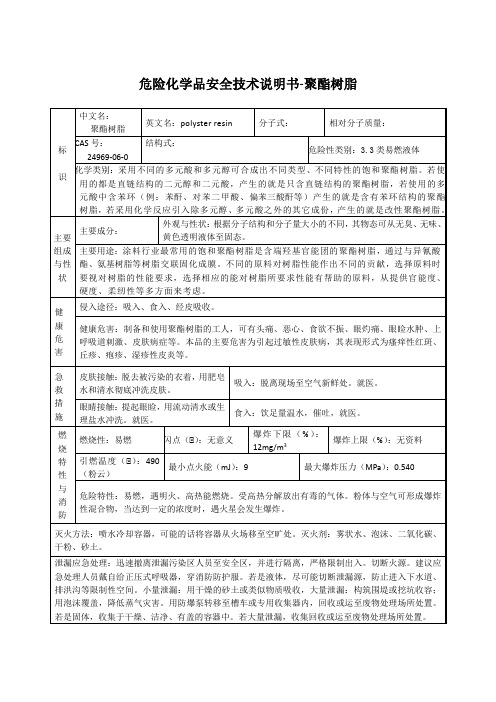

环己胺安全技术说明书第一部分:化学品名称产品目录编号:294化学品中文名:环己胺,六氢苯胺化学品英文名:cyclohexylamine, hexahydroaniline第二部分:成分/组成信息有害成份:环己胺含量: ≥98.5%CAS No:08-91-8分子式:C6H13N分子量:92.19第三部分:危害性概述侵入途径:吸入、食入、经皮吸收。
健康危害:吸入本品蒸气可发生急性中毒。
中毒表现有剧烈呕吐及腹泻;瞳孔散大和对光反应迟钝、视力模糊、萎靡、语言障碍。
人体斑贴试验见25%本品溶液引起严重的皮肤刺激,并可能致过敏反应。
环境危害:对水体、土壤和大气可造成污染。
爆炸危险:本品易燃,具强腐蚀性、强刺激性,可致人体灼伤,具致敏性。
第四部分:急救措施皮肤接触:脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
如有不适感,就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
如有不适感,就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
呼吸、心跳停止,立即进行心肺复苏术。
就医。
食入:饮水,禁止催吐。
如有不适感,就医。
第五部分:消防措施危险特性:易燃,遇明火、高热易燃。
受热分解释出剧毒的烟雾。
与氧化剂接触猛烈反应。
其蒸气比空气重,能在较低处扩散到相当远的地方,遇火源会着火回燃。
有害燃烧产物:一氧化碳、二氧化碳、氧化氮。
灭火方法:用水喷射逸出液体,使其稀释成不燃性混合物,并用雾状水保护消防人员。
灭火剂:水、抗溶性泡沫、干粉、二氧化碳、砂土。
第六部分:泄漏应急处理迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿防酸碱工作服。
从上风处进入现场。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
小量泄漏:用砂土、干燥石灰或苏打灰混合。
也可以用大量水冲洗,洗水稀释后放入废水系统。
大量泄漏:构筑围堤或挖坑收容。
用泡沫覆盖,降低蒸气灾害。
用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。

异氰酸环己酯
环己基异氰酸酯安全技术说明书
说明书目录
第一部分化学品名称第九部分理化特性
第二部分成分/组成信息第十部分稳定性和反应活性第三部分危险性概述第十一部分毒理学资料
第四部分急救措施第十二部分生态学资料
第五部分消防措施第十三部分废弃处置
第六部分泄漏应急处理第十四部分运输信息
第七部分操作处置与储存第十五部分法规信息
第八部分接触控制/个体防护第十六部分其他信息
第一部分:化学品名称
化学品中文名称:异氰酸环己酯
化学品俗名:
环己基异氰酸酯
化学品英文名称:Cyclohexyl
isocyanate;
英文名称:
Isocyanic acid,
cyelohexyl ester
技术说明书编码:CAS No.: 3173-53-3
生产企业名称:
地址:
生效日期:
第二部分:成分/组成信息
第三部分:危险性概述
危险性类别:
侵入途径: 吸入食入经皮吸收
有毒。
吸入、摄入或经皮肤吸收后会中毒。
强烈刺激和腐蚀皮肤、眼睛和粘膜。
可引起过敏
健康危害:
反应。
接触后,出现烧灼感、头痛、头晕、咳嗽、气短、恶心、呕吐等,长时间接触,可引起哮喘。
环境危害: 燃爆危险:
消防器材。
储存间内的照明、通风等设施应采用防爆型,开关设在仓外。
搬运时要轻装轻卸, 防止包装及容器损坏。
分装和搬运作业要注意个人防护。
运输按规定路线行驶。
辛醇/水分配系数的对数值:
闪点(C ):35 爆炸上限%(V/V): 引燃温度(C ):爆炸下限%(V/V): 溶解性:微溶于水。
主要用途:用于有机合成。
其它理化性质:
第十部分:稳定性和反应活性
稳定性:稳定
禁配物:强氧化剂、强碱、水、酸类、醇类、胺类。
避免接触的条件:受热、接触潮湿空气。
聚合危害:不能出现
分解产物:一氧化碳、二氧化碳、氮氧化物、氰化氢。
第十一部分:毒理学资料
急性毒性:LD50 : LD50 : 13mg / kg(小鼠腹腔)LC50 :
LC50 :
亚急性和慢性毒性:
刺激性:
致敏性:
致突变性:
致畸性:
致癌性:
第十二部分:生态学资料
生态毒理毒性:
生物降解性:
非生物降解性:
生物富集或生物积累性:
其它有害作用:
第十三部分:废弃处置废弃物性质:
废弃处置方法:
废弃注意事项:
第十四部分:运输信息危险货物编号:
UN编号: 2488
包装标志:
包装类别:
包装方法:
运输注意事项:
第十五部分:法规信息
法规信息
第十六部分:其他信息参考文献:
填表部门:
数据审核单位: msds查询网整理
修改说明:
其他信息:。