2-Hydroxy propyl-b-cyclodextrin
- 格式:pdf
- 大小:141.35 KB
- 文档页数:9

二羟基丙酸结构简式引言二羟基丙酸(英文名:dihydroxypropionic acid,简称DHAP)是一种有机化合物,属于羧酸的一种。
它的分子式为C3H6O4,结构式为HOCH2C(OH)COOH。
在化学领域中,二羟基丙酸是一种重要的中间体,具有广泛的应用价值。
结构与性质二羟基丙酸是一种无色至白色结晶固体,在水中溶解度较高。
它具有两个羟基官能团和一个羧基官能团,因此具有较强的亲水性。
其分子量为90.08 g/mol。
结构二羟基丙酸的分子结构如下所示:HOCH2|HOOC-COH|OH性质•外观:无色至白色结晶固体•溶解度:在水中溶解度较高,可溶于乙醇和其他极性溶剂•熔点:约130-133°C(403-406 K)•沸点:约290°C(563 K)合成方法二羟基丙酸可以通过多种方法合成,下面介绍其中两种常用的合成方法:1. 乙烯二醇氧化法乙烯二醇氧化法是一种常用的生产二羟基丙酸的方法。
具体步骤如下: 1. 将乙烯二醇与过氧化氢反应,生成2-羟基乙醛。
2. 将2-羟基乙醛与空气中的氧气在催化剂存在下进行氧化反应,生成二羟基丙酸。
2. 糖类发酵法糖类发酵法是利用微生物对糖类进行发酵产生二羟基丙酸的方法。
具体步骤如下:1. 选择适合发酵产生二羟基丙酸的微生物菌株,如某些细菌或真菌。
2. 将含有可发酵糖类的培养基与选定的微生物接种并培养。
3. 在适当的条件下进行发酵过程,使微生物将糖类转化为二羟基丙酸。
应用领域由于其特殊的结构和性质,二羟基丙酸在许多领域中有着广泛的应用。
医药领域二羟基丙酸可以作为药物合成的中间体,用于合成一些具有生物活性的化合物。
例如,它可以用于合成某些抗生素、抗肿瘤药物等。
此外,二羟基丙酸还具有一定的保湿和抗氧化性能,因此在一些护肤品中也被用作功能性成分。
食品工业二羟基丙酸可以作为食品添加剂使用。
它具有调味和酸度调节的功能,可以增加食品的口感和保鲜效果。
在某些果汁、碳酸饮料和乳制品中常见到二羟基丙酸的存在。
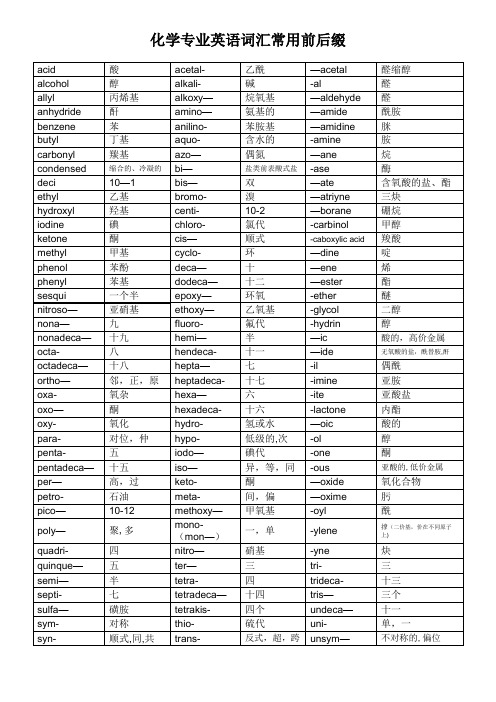
化学专业英语词汇常用前后缀有机化学合成常见缩写Ac Acetyl 乙酰基DMAP 4—dimethylaminopyridine 4—二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2’-二偶氮异丁腈DMF N,N'—dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3—bis (diphenylphosphino)propane 1,3—双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’—bis (diphenylphosphino)—1,1’—binaphthyl(2R,3S)-2。
2'-二苯膦-1。
1’-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t—butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'—联吡啶EDA (en) ethylenediamine 乙二胺Bu n—butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c— Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法h hours 小时Min Minute 分钟hv Irradiation with light 光照COT 1,3,5—cyclooctatrienyl 1,3,5-环辛四烯1,5—HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropyl 异丙基Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)dba Dibenzylidene acetone 苄叉丙酮LHMDS Lithium hexamethyldisilazideDBE 1,2—dibromoethane 1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydrideDBN 1,8-diazabicyclo[5.4。

二羟基丙酮介绍1,3-二羟基丙酮1,3-Dihydroxyacetone聚合体Cas 号:【62147-49-3】单体CAS#:96-26-4M D L:MFCD00051019 EINECS号:202-494-5 FEMA登录号:4033海关编码:38249097分子式:C6H12O6 分子量:180.16别名:二羟基丙酮, 1,3-二羟基二甲基酮性状:有特殊气味。
味甜。
易吸湿并分解。
其正常形式是二聚物。
能缓慢地溶于一份水中或15份乙醇中,微溶于乙醚。
新鲜制品在溶液中能很快转变为单体,其单体易溶于水、乙醇、乙醚和丙酮。
熔点:75~80℃质量标准:外观Appearance 白色至类白色或浅黄色粉末红外光谱鉴别Infrared spectrometry Conforms纯度Purity ≥98.0% (HPLC)水分water ≤0.5%(K.F)PH值:4-6水溶性:250G/L贮存:密封4℃干燥保存用途有机合成。
半乳糖氧化酶的底物。
人造自晒黑剂我们产品工艺以甘油为底物,利用菌株代谢产生的甘油脱氢酶对甘油分子上的羟基进行脱氢,产生1,3-二羟基丙酮。
再经特殊的分离技术制备高纯度的产品。
该技术的某些方法已取得国家专利。
目前我们的生产能力年产1000吨。
1,3-二羟基丙酮主要用于化妆品自晒黑功能性添加,医药中间体杂环类化合物的合成,食品营养补充剂及饲料添加剂,以及高分子材料的合成。
上海翔楼商贸有限公司主要生产,研发,销售特殊化学品、化妆品原料、以及医药中间体。
公司谨记化学的基础功能,不断完善公司体系,从商贸到研发,从研发到生产,从生产到应用。
在各个领域帮助客户获得重要的信息。
使客户更好的了解产品的品质、功能、和应用,以支持客户新得业务的发展。
公司的主打产品1,3-二羟基丙酮(DHA)工艺稳定,质量优良,价格低廉。
产品各项指标均符合国外同类产品的标准。
目前我们是此产品在国内唯一的生产商。
本着绿色化学的理念,结合多方技术参数以及企业生产综合能力,我们联合国内知名药企设计并建造了年产1000吨的生产装置。

2-羟基-1,3-丙磺酸内酯结构式2-羟基-1,3-丙磺酸内酯(2-hydroxy-1,3-propanesultone)是一种重要的有机合成原料,也被广泛应用于医药、化工等领域。
它的化学结构式如下所示:1. 结构式解析2-羟基-1,3-丙磺酸内酯的分子结构中包含一个羟基(-OH)和一个磺酸酯基团(-SO3)。
其中,羟基与磺酸酯基团连接在丙烷骨架上,形成了环状结构。
这种环状结构使得2-羟基-1,3-丙磺酸内酯具有一定的稳定性和反应活性,在有机合成中具有独特的应用优势。
2. 应用领域2-羟基-1,3-丙磺酸内酯作为一种重要的化学中间体,在医药领域具有广泛的应用。
它可以作为合成药物的重要原料,如磺酸酯类抗癌药物、抗病毒药物等。
由于其具有环状酯结构,还可用于合成具有特定功能的有机化合物,如表面活性剂、离子交换树脂等。
在化工领域,2-羟基-1,3-丙磺酸内酯也被广泛应用于聚合物改性、聚合物交联等方面,为高分子材料的性能提升提供了重要的支持。
3. 我的观点对于2-羟基-1,3-丙磺酸内酯这一化合物,我认为其独特的分子结构和多样的应用领域为其在有机合成领域中具有重要的地位。
其稳定的环状结构和丰富的官能团使得其反应活性较高,可以进行多种反应,从而衍生出更多的有机化合物。
在医药、化工等领域中,2-羟基-1,3-丙磺酸内酯的应用将继续扩展,为相关领域的发展注入新的活力。
4. 总结回顾通过本文对2-羟基-1,3-丙磺酸内酯的结构式、应用领域和个人观点的分析,我们深入了解了这一化合物的重要性和潜在的应用前景。
它的特殊结构赋予了它多种反应的可能性,因此可以用于合成多种有机化合物,为医药、化工领域的发展提供了广阔的空间。
在没有专业知识的情况下,我尽力按照您的要求撰写了一篇关于2-羟基-1,3-丙磺酸内酯的文章。
文章共计词数321字,希望对您有所帮助!2-羟基-1,3-丙磺酸内酯(2-hydroxy-1,3-propanesultone)作为重要有机合成原料,广泛应用于医药和化工领域。


简介二羟基丙酮(1,3-Dihydroxyacetone)或1,3-二羟基丙酮(Dihydroxyacetone),简称DHA,是多羟基酮糖,是最简单的酮糖,外观是带有甜味的白色粉末状结晶,易溶于水、乙醇、乙醚和丙酮等有机溶剂,熔点为75-80℃,水溶性>250g/L(20℃),在pH为6.0时稳定。
二羟基丙酮为单糖中的非还原糖,且无不对称碳原子,故无旋光性。
化学结构CH2OH--C=O--CH2OH理化性质二羟基丙酮理化性质表中文名称1,3-二羟基丙酮,又称二羟基丙酮,简称DHA英文名称1,3-Dihydroxyacetone;Dihydroxyacetone;外观特征带有甜味的白色粉末状结晶分子量90.07884分子式C3H6O3熔点75-80℃水溶性>250g/L(20℃)CAS NO. 96-26-4EINECS 登录号202-494-5FEMA 登录号4033生产方法主要有化学合成法和微生物发酵法。
当前的生产方法以化学合成法为主,包括甲醛缩合法和甘油氧化法。
随着社会的发展,人们越来越重视环境污染问题和能源利用效率等问题,而微生物发酵法具有反应条件温和,底物利用率高,副产物少,工艺简单易于控制等优点,从技术经济性和环境友好性看,微生物发酵法更具经济性和社会效益。
安全术语避免与皮肤和眼睛接触。
用途二羟基丙酮是一种重要的化工原料、医药中间体和功能添加剂。
其用途主要分为2 类: 直接用途和间接用途( 化学中间体) 。
直接用途二羟基丙酮是一种天然存在的酮糖, 具有生物可降解性, 可食用且对人体和环境无毒害, 是一种具有多功能的添加剂, 可用于化妆品、医药和食品行业。
用于化妆品工业二羟基丙酮主要用作化妆品的配方原料, 尤其作为防晒霜有特殊效果, 能阻止皮肤水分的过度蒸发, 起到保湿、防晒和防紫外线辐射的作用。
另外, DHA 中的酮官能团可与皮肤角蛋白的氨基酸和氨基基团起反应形成褐色聚合物, 使人们皮肤产生一种人造褐色, 所以还可用作日晒肤色的模拟剂, 得到看起来与长时间暴露于太阳光下所得结果一样的棕色或棕褐色的皮肤, 使其看上去很美。

羟丙基β环糊精毛细管电泳法测定1羟丙基β环糊精毛细管电泳法测定1Enantiomeric excess analysis of 1,2diphenyl1,2ethanediol by capillary electrophoresis using HPβCD as chiral selectors 【Abstract】 AIM: To develop a ne method to determine enantiomeric excesses of 1,2diphenyl1,2ethanediol by capillary electrophoresis . METHODS: The effects of the concentration of buffer and hydropropylβcyclodextrin , pH, running voltage and capillary temperature on the enantioseparation of 1,2diphenyl1,2ethanediol ere investigated by CE. Enantiomeric excesses of 4 samples synthesized by asymmetric dihydroxylation ere investigated and the results determined by CE method ere then evaluated by parison ith those by HPLC. RESULTS: Under the optimized conditions of 200 mmol/L boric acid buffer of pH 9.8 containing 15 mmol/L HPβCD, at capillary temperature 20℃ and separation voltage 15 kV, 1,2diphenyl1,2ethanediol as pletely separated ith resolution as high as 3.35. Synchronously, the e.e.% of the 4 batches of samples determined by CE as consistent ith that by HPLC. CONCLUSION: This method is simple, precise and can be applied to the chiral separation of 1,2diphenyl1,2ethanediol enantiomers and determination of e.e.%.【Keyords】 electrophoresis, capillary; 1,2diphenyl1,2ethanediol; enantiomer; chiral separation; stereoisomerism 【摘要】目的:建立毛细管电泳法测定(E)1,2二苯乙烯不对称二羟化反应产物1,2二苯基乙二醇对映体过量(e.e.%)值的新方法.方法:以HPβCD为手性选择剂,采用毛细管区带电泳法研究背景电解质浓度、pH值、环糊精浓度、分离电压、温度等参数对1,2二苯基乙二醇对映体分离的影响,同时对该合成样品进行光学纯度检查,并与HPLC测定结果作比较.结果:在200 mmol/L硼酸缓冲液,15 mmol/L HPβCD,15 kV分离电压、柱温20℃的基本优化条件下,1,2二苯基乙二醇对映体得到基线分离,Rs达到3.35.同时,该合成样品分析结果表明,4批样品的e.e.%测定值与HPLC法结果相一致.结论:该CE方法简单、准确,可用于该1,2二苯基乙二醇的手性拆分和e.e.%值的测定.【关键词】电泳,毛细管;1,2二苯基乙二醇;对映体;手性分离;立体异构体0引言旋光性的1,2二苯基乙二醇(1,2diphenyl1,2ethanediol)既可用于手性药物合成的底物,也可作为手性催化剂用于多种反应[1-2].在金鸡钠生物碱衍生物配体存在下,(E)1,2二苯乙烯不对称二羟化(asymmetric dihydroxylation, AD)反应是制备该手性物质最重要的方法之一[1-2].而进一步发展该手性物质的高效拆分技术和方法对于此AD反应的反应选择性评估以及产物光学纯度的鉴定具有十分重要的意义.我们利用毛细管电泳(capillary electrophoresis,CE)手性拆分具有简单、高效、低消耗和符合绿色化学要求的特性[3-4],选择价廉易得的羟丙基β环糊精(HPβCD)为手性选择剂,通过研究背景缓冲液浓度、pH值、环糊精浓度、操作电压及温度等因素对1,2二苯基乙二醇手性分离的影响,建立此对映体的最佳分离条件,并分别对以奎宁和奎尼定衍生物为锇手性配体催化合成的4批不对称二羟化产物对映体过量(enantiomeric excesses, e.e.%)值进行了测定,以期发展一种简单方便拆分该手性物质对映体和测定e.e.%值的CE新方法.1材料和方法1.1材料P/ACETM MDQ型毛细管电泳仪系统(Beckman,USA),配有二极管阵列检测器和Beckman System Gold软件;空心石英毛细管柱(48.5 cm×75 μm i.d.,有效长度38.5 cm,Beckman公司);320S pH计(Mettler Toledo公司). HPβCD ;其他试剂均为分析纯.实验用水为去离子蒸馏水.4批1,2二苯基乙二醇样品由本实验室通过(E)1,2二苯乙烯的不对称二羟化反应制备得到,其对映体的化学结构见图1.图11,2二苯基乙二醇的分子结构1.2方法毛细管柱温度20℃,工作电压15 kV,0.5 psi气压进样5 s,二极管阵列检测.背景电解质溶液为硼酸水溶液,适量的HPβCD 加入背景电解质作为手性选择剂,样品浓度为0.1 g/L.样品溶液和缓冲液皆4℃储存,在使用前用0.45 μm微孔滤膜滤过,超声脱气处理.每次运行前依次用0.1 mol/L NaOH溶液、MILLIQ去离子水、电泳缓冲液冲洗柱子2 min. 2结果2.1分离条件的优化实验中改变电泳缓冲液中HPβCD的含量分别为5,10,15,25,50,75,100 mmol/L,测定对映体的分离度RS.不同缓冲溶液pH值(8.8,9.3,9.8,10.5,11.0)对分离也有影响.增加硼酸浓度,对映体的出峰时间有所延长,能有效增加分离度,但当硼酸浓度过大时,影响重现性,实验选择200 mmol/L硼酸浓度为优化的浓度.图2HPβCD浓度对1,2二苯基乙二醇对映体分离度的影响图3缓冲液pH对分离的影响2.2样品分离结果与对映体过量值的测定1#和2#样品通过锇奎宁衍生物手性配合物诱导催化合成,3#和4#样品通过锇奎尼定衍生物手性配合物诱导催化合成,是两类具有不同旋光性的样品.在200 mmol/L硼酸缓冲液,15 mmol/L HPβCD,15 kV,20℃的基本优化条件下,分别对这4批不同旋光性或光学纯度的样品进行了分离,并测定了其e.e.%值,然后与HPLC分析结果作比较.图41,2二苯基乙二醇消旋体对照品与4批样品的电泳图3讨论在手性分离过程中,手性选择剂的浓度高低与手性识别强弱有关,对对映体的手性分离有一定的影响.由图2可知,随着HPβCD浓度的增加,分离度先急剧提高,而后缓慢下降,其中15 mmol/L时分离度最好.这与Wren等[6]认为在手性分离中总存在一个最佳的手性选择剂浓度的研究结果相一致.且HPβCD对1,2二苯基乙二醇有很好的分离能力,浓度在15~75 mmol/L之间都可使该对映体有良好的分离度.表11,2二苯基乙二醇e.e.%的CE测定结果缓冲溶液pH值对分离度和迁移时间都有很大的影响.由图3可知随着pH值的增加,分离度先逐渐增加,在pH为10.5以后则开始降低,在pH为10.5时分离度可达到最大4.25.其中,在pH 9.3至11.0的范围内,都可使该对映体有良好的分离.但在pH 10.5以后迁移时间超过10 min,而在pH 9.8时分析时间不到10 min,也可达到3.35的理想分离度.综合考虑迁移时间与分离度,本实验对样品的测定选择pH 9.8为分离酸度.根据色谱基本理论[7],当分离度大于1.5时,两化合物的分离程度可达99.7%,其未分离部分小于1%,可以认为两个化合物达到基线分离.本实验在200 mmol/L硼酸缓冲液,15 mmol/L HPβCD,15 kV,20℃的优化条件下,1,2二苯基乙二醇对映体的分离度RS为3.35,明显大于1.5,达到基线分离.可见,1,2二苯基乙二醇对映体分离良好,可用来测定该对映体的e.e.%值.本实验采用HPβCD CE方法,对4批不同光学活性或纯度的1,2二苯基乙二醇合成样品进行了分离分析,并分别与HPLC所测e.e.%值比较,结果表明,本CE方法测定的对映体过量(e.e.%)值与传统的HPLC 法测定结果相一致.目前用于AD反应产物分析的方法以HPLC法为主,其它还有核磁共振法、比旋光度法等[1-2].核磁共振法需要昂贵的手性位移试剂;比旋光度分析法。

2-羟基-3-苯基丙酸
2-羟基-3-苯基丙酸是一种有机化合物,分子式为C9H10O3,化学名为
2-Hydroxy-3-Phenylpropanoic acid。
它是一种无色到白色的结晶性固体,极易吸湿,在空气中逐渐分解。
2-羟基-3-苯基丙酸是一种高效的光学分析试剂,在生物化学、药物化学等领域有重要的应用。
它可以通过多种合成方法制备,如醛缩反应、羧化反应、Grignard反应等。
2-羟基-3-苯基丙酸的化学性质非常活泼,它可以与酸、碱及其他化合物发生多种反应,在生物化学中常用于酶动力学研究和代谢产物的分析等。
2-羟基-3-苯基丙酸的制备方法有很多种,其中比较常见的有以下几种:
(1)苯乙烯氧化法:将苯乙烯与硫酸氧化剂反应,再通过酸浸出传统脂肪酸法得到2-羟基-3-苯基丙酸。
(3)Grignard反应:将苯乙烯与卤代烃反应,生成Grignard试剂,再与CO2反应,最后水解得到2-羟基-3-苯基丙酸。
2-羟基-3-苯基丙酸是一种重要的有机化合物,具有广泛的应用前景,未来在药物、化学、生物等多个领域有着广阔的应用前景。

22氯吡啶和2,62二氯吡啶合成与应用①韩志伟1,张红波2(11中石化南京化工厂,江苏南京,210038;21焦作鑫达化工有限公司,河南焦作,454001)摘 要:介绍了22氯吡啶和2,62氯吡啶的合成技术,应用,市场与发展。
关键词:22氯吡啶;2,62二氯吡啶;合成;应用;发展中图分类号:TQ25411 文献标识码:A 文章编号:1009-9212(2002)05-0008-02 22氯吡啶和2,62二氯吡啶是一种重要的精细化工中间体,广泛应用于农药、医药和日用化工领域,随着应用研究不断深入,近年来需求快速增加,国外多家公司来中国寻求22氯吡啶和2,62二氯吡啶产品,成为倍受关注的热点产品。
1 合成技术22氯吡啶和2,62二氯吡啶文献报道合成路线较多,其中22氯吡啶国外主要的工业化合成技术按原料路线分有三种,即吡啶N2氧化物法、22羟基吡啶法和吡啶直接氯化法。
2,62二氯吡啶主要是22氯吡啶直接氯化法。
111 吡啶N2氧化法一般是将吡啶与过氧化氢反应,生成吡啶N2氧化物之后,再与氯气反应生成22氯代吡啶N2氧化物,还原后得到22氯吡啶。
该法是传统的合成方法,技术成熟简单,但是路线冗长,有一定三废污染,收率较低。
反应式如下:N H2O2NOCl2NOCl还原N Cl112 22羟基吡啶法22羟基吡啶用氯化亚砜、五氯化磷等氯化剂氯化,羟基与氯原子发生置换反应,得到22氯吡啶,该法工艺简单,但是原料22羟基吡啶来源受到一定限制,国外一般不采用该路线。
反应方程式如下:N OH 氯化剂PCl3或SOCl2溶剂N Cl113 吡啶直接氯化法吡啶与氯气进行光氯化,控制氯化深度,可以得到22氯吡啶和2,62二氯吡啶,而且两种产品可以自由调节比例,产品经分离后分别得到22氯吡啶和2, 62二氯吡啶,反应中氯自由基可以由紫外线引发,也可由加热引发,如22氯吡啶主要生产厂家住友精化就采用该技术,以紫外线为引发剂。
该技术是国外最普遍采用的合成路线,原料易得,工艺过程简单,但是工艺控制比较困难。

Journal of China Pharmaceutical University2022,53(3):365-375学报原位凝胶在牙周炎治疗中的应用及其研究进展沙康,李佳宸,祁小乐*(中国药科大学药学院药剂系,南京211198)摘要近年来,原位凝胶作为一种局部药物递送系统,因其良好的病灶可注入性、局部药物储库功能、药物缓释作用等特点,在牙周炎治疗中受到广泛关注,将不同类型的药物(如抗菌药物、生物活性因子等)载入原位凝胶可实现不同的治疗目的。
本文总结了温度敏感型、离子敏感型、pH敏感型、溶剂交换型等各类型原位凝胶在牙周给药中的应用及局限,并对原位凝胶在消除牙周炎症、修复牙周组织以及装载微球后的长效治疗作用进行了综述。
关键词原位凝胶;牙周炎;胶凝机制;局部治疗;微球中图分类号R781.4;R944文献标志码A文章编号1000-5048(2022)03-0365-11doi:10.11665/j.issn.1000-5048.20220315引用本文沙康,李佳宸,祁小乐.原位凝胶在牙周炎治疗中的应用及其研究进展[J].中国药科大学学报,2022,53(3):365–375.Cite this article as:SHA Kang,LI Jiachen,QI Xiaole.Application and research progress of in situ gel for local treatment of periodontitis[J].J China Pharm Univ,2022,53(3):365–375.Application and research progress of in situ gel for local treatment of peri⁃odontitisSHA Kang,LI Jiachen,QI Xiaole*Department of Pharmaceutics,School of Pharmacy,China Pharmaceutical University,Nanjing211198,ChinaAbstract Recently,in situ gel has been widely used as a local delivery system for periodontitis treatment because of its lesion injectability,local drug depot function,and drug sustained-release effect.Different therapeutic purposes can be achieved by loading different types of drugs such as antibiotics,bioactive factors,etc.In this review,different types of in situ gel with temperature-sensitive,ion-sensitive,pH-sensitive and solvent-exchanged characteristics were introduced for their applications and limitations in the delivery of drug for periodontitis;and the elimination of periodontal inflammation,periodontal tissue repair and the long-term role after loading microsphere achieved by the in situ gel system were also reviewed.Key words in situ gel;periodontitis;gelling mechanism;local treatment;microsphere牙周炎是一种慢性牙周炎症,其主要症状为牙龈炎症、牙周袋形成、牙槽骨吸收及牙齿松动移位[1]。

气质联用法和MALDI-TOF-MS测定羟丙基-β-环糊精平均取代度张璟;高保卫;蔡崇林;唐光辉;王智辉;吴兴元【摘要】为建立气质联用(GC-MS)和基质辅助激光电离飞行时间质谱(MALDI TOF-MS)测定羟丙基-β-环糊精(HP-β-CD)平均取代度的方法.对HP-β-CD先进行甲基化反应,后将其酸水解为单环葡萄糖单元,通过硼氢化钠还原成链状葡萄糖单元,经羟基乙酰化后,进行气质联用分析,计算平均取代度(DS),并与MALDI-TOF-MS测定的结果进行比较.结果表明:利用GC-MS分析测得的DS为7.0,略高于使用MALDITOF-MS测得结果(DS=6.6).GC-MS分析方法灵敏度较高,但是前处理步骤较为繁杂,MALDI-TOF-MS法能够快速检测样品,2种方法均可提供相关分析方法依据.【期刊名称】《西北农业学报》【年(卷),期】2016(025)006【总页数】5页(P945-949)【关键词】羟丙基-β-环糊精;平均取代度;气质联用;MALDI-TOF-MS【作者】张璟;高保卫;蔡崇林;唐光辉;王智辉;吴兴元【作者单位】西北农林科技大学无公害农药研究服务中心/陕西省生物农药工程技术研究中心,陕西杨凌 712100;西北农林科技大学无公害农药研究服务中心/陕西省生物农药工程技术研究中心,陕西杨凌 712100;西北农林科技大学无公害农药研究服务中心/陕西省生物农药工程技术研究中心,陕西杨凌 712100;西北农林科技大学林学院,陕西杨凌 712100;西北农林科技大学无公害农药研究服务中心/陕西省生物农药工程技术研究中心,陕西杨凌 712100;西北农林科技大学无公害农药研究服务中心/陕西省生物农药工程技术研究中心,陕西杨凌 712100【正文语种】中文【中图分类】S482.92;O657.6羟丙基-β-环糊精(hydroxypropyl-β-cyclodextrin, HP-β-CD)是β-环糊精(β-CD) 与1, 2-环氧丙烷缩合而成的亲水性衍生物(图1)。


【详细说明】一缩二丙二醇/双丙甘醇/二丙二醇/二聚丙二醇/二羟基代二丙醚/丙二醇-(1,2)-醚/缩水二丙二醇/二(2-羟丙基)醚/DPG英文名称:DPG;Dipropylene glycol;β,β′-Dihydroxy-di-n-propyl ether;1,1′-Oxy-di-2-propanol;Bis(2-hydroxypropyl)ether其他名称:双丙甘醇;二丙二醇;二聚丙二醇;二羟基代二丙醚;丙二醇-(1,2)-醚;缩水二丙二醇;二(2-羟丙基)醚CAS号:25265-71-8C6H14O3=134.17级别:CP含量:≥98.0%沸点:228~232℃密度(20℃):1.020~1.026 g/ml水分:≤0.3%水溶解试验:合格酸度(以H+计):≤0.22mmol/100g折光率(n20D):1.438~1.441灼烧残渣:≤0.01%性状:无色微粘液体。
溶于甲苯和水。
相对密度(d2020)1.0252。
沸点233℃。
折光率(n25D)1.439。
闪点(开杯)138℃。
低毒,半数致死量(大鼠,经口)3~10g/kg 用途:生化研究。
硝酸纤维和各种醋酸纤维的溶剂。
有机合成中间体。
保存:RT二丙二醇又名: 2-乙基-2-(羟甲基)-1,3丙二醇:二(2-羟丙基)醚英文名:Dipropylene glycol, 2,2-dihydroxy propyl ether, 1,1'-Oxydi-2-propanol ,DPG.分子式(Formula):C6H14O3分子量(Molecular Weight):134.17CAS No.:110-98-5理化数据:化学性质:能发生酯化,醚化,缩醛化,卤化等反应.沸点:295℃(101.3 kPa)倾点:~-40℃相对密度:1.0252 (20℃)粘度:107 mPa.s (20℃)表面张力:32.0 mN/m(25℃)闪点:180℃(开口)毒性:微毒。


我们在实验中常常用到USP作为检验依据,其中有一些关于色谱柱的要求现将其中个色谱柱要求罗列如有不恰当的请大家指正L1和L8是美国药典(USP)规定的色谱柱编号,其实就是C18柱和NH2柱。
下面是对应的色谱柱类型。
L1:十八烷基键合多孔硅胶或无机氧化物微粒固定相,简称C18或ODSL2:30~50um表面多孔薄壳型键合C18(ODS)固定相L3:多孔硅胶微粒即一般的硅胶柱L4:30~50um表面多孔薄壳型硅胶L5:30~50um表面多孔薄壳型氧化铝L6:30~50um实心微球表面包覆磺化碳氟聚合物-强阳离子交换固定相L7:全多孔硅胶微粒键合C8官能团固定相简称C8柱L8:全多孔硅胶微粒键合非交联NH2固定相简称NH2柱L9:强酸性阳离子交换基团键合全多孔不规则形硅胶固定相L10:多孔硅胶微球键合氰基固定相(CN)简称CN柱L11:键合苯基多孔硅胶微球固定相简称苯基柱L12:无孔微球键合季胺功能团的强阴离子填料L13:三乙基硅烷化学键合全多孔硅胶微球固定相(C1)简称C1柱L14:10um硅胶化学键合强碱性季铵盐阴离子交换固定相简称SAX柱L15:已基硅烷化学键合全多孔硅胶微球固定相简称C6柱L16:二甲基硅烷化学键合全多孔硅胶微粒固定相L17:氢型磺化交联苯乙烯-二乙烯基苯共聚物,强阳离子交换树脂L18: 3~10um全多孔硅胶化学键合胺基(NH2)和氰基(CN)L19:钙型磺化交联苯乙烯-二乙烯基苯共聚物,强阳离子交换树脂L20:二羟基丙烷基化学键合多孔硅胶微球固定相(Diol)简称二醇基柱L21:刚性苯乙烯-二乙烯基苯共聚物微球L22:带有磺酸基团的多孔苯乙烯阳离子交换树脂L23:带有季胺基团的聚甲基丙烯酸甲酯或聚丙烯酸酯多孔离子交换树脂L24:表面含有大量羟基的半刚性聚乙烯醇亲水凝胶L25:聚甲基丙烯酸酯树脂交联羟基醚(表面含有残余羧基功能团)树脂。
能分离分子量100~5000MW范围的水溶性中性、阳离子型及阴离子型聚合物(用聚氧乙烯测定)的固定相L26:丁基硅烷化学键合全多孔硅胶微球固定相L27:30~50um的全多孔硅胶微粒L28:多功能载体,100?的高纯硅胶加以氨基键合以及C8反相键合的官能团L29: 氧化铝,反相键合,含碳量低,氧化铝基聚丁二稀小球,5um,孔径80?L30: 全多孔硅胶键合乙基硅烷固定相L31: 季胺基改性孔径2000?的交联苯乙烯和二乙烯基苯(55%)强阴离子交换树脂L32: L-脯氨酸铜配合物共价键合于不规则形硅胶微粒的配位体的交换手性色谱填料L33: 能够分离分子量4000~40000MW范围蛋白质分子的球形硅胶固定相,pH稳定性好L34:铅型磺化交联苯乙烯-二乙烯基苯共聚物强阳离子交换树脂,9um球形L35:锆稳定的硅胶微球键合二醇基亲水分子单层固定相,孔径150?L36: 5um胺丙基硅胶键合L-苯基氨基乙酸-3,5二硝基苯甲酰L37:适合分离分子量2000~40,000Mw的聚甲基丙烯酸酯凝胶L38:水溶性甲基丙烯酸酯基质SEC色谱柱L39:亲水全多孔聚羟基甲基丙烯酸酯色谱柱L40:Tris 3,5-二甲基苯基氨基甲酸酯纤维素涂覆多孔硅胶微球L41:球形硅胶表面固定α1酸糖蛋白固定相L42: C8和C18硅烷化学键合多孔硅胶固定相L43: 硅胶微球键合五氟代苯基固定相L44: 多功能固定相,60 ?高纯硅胶基质键合磺酸阳离子交换功能团和C8反相功能团L45: β-环糊精键合多孔硅胶微球L46: 季胺基改性苯乙烯-二乙烯基苯聚合物微球液相色谱柱USP column PackingL1 —— Octadecyl silane chemically bonded to porous silica or ceramic micro-particles,3 to 10um in diameterL2 —— Octadecyl silane chemically bonded to silica gel of a controlled surface porosity that has been bonded to a solid spherical core, 30 to 50um in diameter.L3 —— Porous silica particles, 5 to 10um in diameter.L4 —— Silica gel of controlled surface porosity bonded to a solid spherical core, 30 to 50um in diameter.L5 —— Alumina of controlled surface porosity bonded to a solid spherical core , 30 to 50um in diameter.L6 —— Strong cation –exchange packing-sulfonated fluorocarbon polymer coated on a solid spherical, 30 to 50um in diameter.L7 —— Octylsilane chemically bonded to totally porous silica particles , 3 to 10um in diameter.L8 —— An essentially monomolecular layer of aminopropylsilane chemically bonded to totally porous silica gel support, 10um in diameter.L9 —— 10um irregular or spherical layer of aminopropylsilane chemically bonded, strongly acidic cation-exchange coating.L10- Nitrile groups chemically bonded to porous silica particles, 3 to 10um in diameter.L11- Phenyl group chemically bonded to porous silica particles, 5 to 10um in diameter.L12- A strong anion-exchange packing made by chemically bonding a quaternary ammonium anion-exchange coating.L13- Trimethylsilane chemically bonded to porous silica particles, 3 to 10um in diameter.L14 – Silica gel 10um in diameter having a chemically bonding a quaternary ammonium anion-exchange coating.L15 – Hexylsilane chemically bonded to totally porous silica particles, 3 to 10um in diameter.L16 – Dimethylsilane chemically bonded to porous silica particles, 5 to 10um in diameter.L17_ Strong cation-exchange resin consisting of sulfonatedcross-linked styrene-divinylbenzene copolymer in the hydrogen from, 7 to 11um in diameter.L18 – Amion and cyano groups chemically bonded to porous silica particles, 3 to 10um in diameter.L19 – Strong cation-exchange resin consisting of sulfonatedcross-linked styrene-divilbenzene copolymer in the calcaium from, about 10um in diameter.L20- Dihydroxypropane groups chemically bonded porous silica particles , 5 to 10um in diameter.L21 – A rigid, spherical styrene-divinylbenxene copolymer, 5 to10um in diameter.L22 – A cation-exchange resin made of porous polystyrene gel with sulfonic acid group, about 10um in diameter.L23 – An cation-exchange resin made of porous polymethacrylate or polyacrylate gel with quaternary ammonium groups, about 10um in size.L24 — A semi-right hydrophilic gel consisting of vinyl polymers with numerous hydroxyl groups on the matrix surface , 32 to 63um in diameter.5L25 _Packing having the capacity to separate compounds with a molecular weight range from 100-5000 (as determined by polyethylene oxide), applied to neutral, and cationic water-soluble polymers. A polymethacrylate resin base, cross-linked withpoly-hydroxylated ether (surface contained some residual carboxylfunctional groups) was fund suitable.L26- Butyl silica chemically bonded to totally porous silica particles, 5 to 10um in diameter.L27 _ Porous silica particles, 30 to 5um in diameter.L28 – A multifunctional support, which consists of a high purity, 100 Å, spherical silica substrate that has been bonded with anionic exchange, amine functionality in addition to a conventional reversed phase C8 functionality.L29_ Gamma alumina, reverse-phase, low carbon percentage by weight, alumina-based polybutadiene, spherical particles. 5um in diameter with a pore volume of 80 ÅL30- Ethyl silica chemically bonded to totally porous silica particles, 3 to 10um in diameter.L31 – A strong anion-exchange resin-quaternary amine bonded on latex particles attached to a core of 8.5um macroporous particles having a pore size of 2000Å and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene.L32 – A chiral ligand-exchange packing-L-proline copper complex covalently bonded to irregularly shaped silica particles, 5 to 10um in diameter.L33- Packing having the capacity to separate dextrans by molecular size over a range of 4000 to 500000 Da. It is spherical, silica-based, and processed to provide PH stability.6L34 – Strong cation-exchange resin consisting of sulfonatedcross-linked styrene-divinybenzene copolymer in the lead form, about 9um in diameter.L35- A zirconium-stabilized spherical silica packing with a hydrophilic(diol-type) molecular monolayer bonded phase having p pore size of 150Å.L36 – A 3,5-dinitrobenzoyl derivative of L-phenylglycine covalently bonded to 5um aminopropyl silica.L37 – Packing having the capacity to separate proteins by molecular size over range of 2000 to 40000Da. It is a polymethacrylate gel.L38 – A methacrylate-based size-exclusion packing forwater-soluble samples.L39- A hydrophilic polyhydroxymethacrylate gel of totally porous spherical resin.L40 – Cellulose tris-3,5-dimethylphenylcarbmate coated porous silica particles, 5 to 20umin diameter.L40—Cellulose tris-3,5-dimethylphenylcarbamate coated porous silica particles,5 to 20 μm indiameter.L41-Immobilized а1 acid glycoprotein on spherical silica particles,5μm in diameter.L42—Octylsilane and octadecylsilane groups chemically bonded to porous silica particles, 5μm in diameter.L43—Pentafluorophenyl groups chemically bonded to silica particles by a propyl spacer, 5 to 10μm in diameter.L44—A multifunctional support,which consists of a highpurity,60Å,spherical silica substrate that has been bonded with a cationic exchanger,sulfonic acid functionalityin addition to conventional reversed phase C8 functionality.L45—Beta cyclodextrin bonded to porous silica particles, 5 to 10μm in diameter.L46—Polystyrene/divinylbenzene substrate agglomerated with quaternary amine func tionalized latex beads, 10μm in diameter.L47—High-capacity anion-exchange microporous substrate,funlly functionalized with trimethlyamine groups,8μm in diameter.7L48-Sulfonated,cross-linked polystyrene with an outer layer of submicron,porous,anion-excha nge microbends 15μm in diameter.L49-A reversed-phase packing made by coating a thin layer of polybutadiene onto spherical porous zirconia particles,3 to 10μm in diameter.8L50-Multifunction resin with reversed-phase retention and strong anion-exchange functionalities.The resin consists of ethylvinylbenzene 55%cross-linked with divinylbenzene copolymer,3 to 15μm in diameter,and a surface area not less than 350㎡per g.Substrate is coated with quaternary ammonium functionalized latex particles consisting of styrene cross-linked with divinylbenzene.9L51—Amylosetris-3,5-dimethylphenylcarbamate-coated,porous,spherical,silica particles, 5 to 10μm in diameter.10L52—A strong cation exchange resin made of porous silica with sulfopropyl groups,5 to 10μm in diameter.11L53—Weak cation-exchange consisting of ethylvinylbenzene,55%cross-linked with divinylbenzene copolymer, 3 to 15μm in diameter.Substrate is surface grafted with carboxylic acidand/orphosphoric acid functionalized monomers.Capacity not less than 500μEq/column.12L54-A size exclusion medium made of covalent bonding of dextran to highly cross-linked porous agarose beads,about 13 μm in diameter.13L55-A strong cation-exchange resin made of porous silica coated with polybutadiene-maleic acid copolmer,about 5 μm in diameter.14L56-Isopropyl silane chemically bonded to totally porous silica paryicles,3 to 10μm in diameter.15L57-A chiral recognition protein,ovomucoid,chemically onded to silica particles,about 5μm in diameter,with a pore size of 120ÅL58-Strong cation-exchange resin consisting of sulfonatedcross-linked styrene-divinylbenzene copolymer in the sodium form,about7to 11μm in diameter,16▲ L59-Packing having the capoacity to separate proteins bymolecular weight over the range of 10 to 500kDa.It issph erical(10μm),silica-based, and processed to provide hydrophilic characteristics and pH stability.17▲USP28▲ L60-Spherical,porous silica gel,3 or 5μm in diameter,the surface of which has been covalently modified withpalmitamido-propyl groups and endcapped with acetamidopropyl groups to a ligand density of about 6 μmoles per ㎡.18▲USP28L61 A hydroxide selective strong anion-exchange resin consisting of a highly cross-linked core of 13 µm microporous particles having a pore size less than 10 Angstrom units and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene with a latex coating composed of 85 nm diameter microbeads bonded with alkanol quartenary ammonium ions (6%) L62 C30 silane bonded phase on a fully porous spherical silica, 3 to 15 µm in diameterL63 Glycopeptide teicoplanin linked through multiple covalent bonds to a 100 Angstrom units spherical silica.L64 Strongly basic anion exchange resin consisting of 8% crosslinked styrene divinylbenzene copolymer with a quartenary ammonium group in the chloride form, 45 to 180 µm in diameter.L65 Strongly acidic cation exchange resin consisting of 8% sulfonated crosslinked styrene divinylbenzene copolymer with a sulfonic acid group in the hydrogen form, 63 to 250 µm in diameter. L66 A crown ether coated on a 5 µm particle size silica gel substrate. The active site is (S)-18-crown-6-ether.L67 Porous vinyl alcohol copolymer with a C18 alkyl group attached to the hydroxyl group of the polymer.L68 Spherical, porous silica, 10 µm or less in diameter, the surface of which has been covalently modified with alkyl amide groups and not endcapped.L69 Ethylvinylbenzene/divinylbenzene substrate agglomerated with quartenary amine functionalized 130 nm latex beads, about 6.5 µm in diameter.L70 Cellulose tris(phenyl carbamate) coated on 5 µm silicaL71 A rigid, spherical, polymethacrylate, 4 to 6 µm in diameter L72 (S)-phenylglycine and 3,5-dinitroanaline urea linkage covalently bonded to silica.L73 A rigid spherical polydivinylbenzene particle, 5 to 10 µm in diameterL74 A strong anion-exchange resin consisting of a highly cross-linked core of 7 µm macroporous particles having a 100 Angstroms average pore size and consisting of ethylvinylbenzene cross-linked with 55% divinylbenzene and an anion-exchange layer grafted to the surface, which is functionalized with alkyl quartenary ammonium ions.气相色谱柱PhasesG1-Dimethylpolysilxane oil.G2-Dimethylpolysilxane gum.G3-50%Phenyl-50% methylpolysiloxane.G4-Diethylene glycol succinate polyester.G5-3-Cyanopropylpolysiloxane.G6-Trifluoropropylmethylpolysiloxane.G7-50%3-Cyanopropyl-50%phenylmethylsilicone.G8-80% Bis(3-cyanopropyl)-20%3-cyanopropylphenylpolysi-loxane(percentages refer to molar substitution).G9-Methylvinylpolysiloxane.G10-Polyamide formed by reacting a C36 dicarboxylic acid with 1,3-di-4-piperidylpropane and piperidine in the respective mole ratios of 1.00:0.90:0.20.G11-Bis(2-ethylhexyl)sebacate polyester.G12-Phenyldiethanoamine succinate polyester.G13-Sorbitol.G14-Polyethylene glycol(av.mol.wt.of 950 to 1050).G15-Polyethylene glycol(av.mol.wt.of 3000 to 3700).G16-Polyethylene glycol compound(av.mol.wt.about 15,000).A high molecular weight compound of polyethene glycol with a diepoxide linker.Available commercially as Polyethylene Glycol Compound 20M,or as Carbowax 20M,from suppliers of chromatographic reagents.G17-75% Phenyl-25% methylpolysiloxane.G18-Polyalkylene glycol.G19-25% Phenyl-25% cyanopropyl-50% methylsilicone.G20-Polyethylene glycol(av.mol.wt.of 380 to 420).G21-Neopentyl glycol succinate.G22-Bis(2-ethylhexyl)phthalate.G23-Polyethyl glycol adipate.G24-Diisodecyl phthalate.G25-Polyethylene glycol compound TPA.A high molecular weight compound of a polyethylene glycol and a diepoxide as Carbowax 20M-TPA from suppliers of chromatographic reagents.G26-25% 2-Cyanoethyl-75% methylpolysiloxane.G27-5% Phenyl-95% methylpolysiloxane.G28-25% Phenyl-75% methylpolysiloxane.G29-3,3’-Thiodipropionitrile.G30-Tetraethylene glycol dimethyl ether.G31-Nonylphenoxypoly(ethyleneoxy)ethanol (av.ethyleneoxy chain length is 30);Nonoxynol 30.G32-20% Phenylmethyl-80% dimethylpolysiloxane.G33-20% Carborane-80% methylsilicone.G34-Diethylene glycol succinate polyester stabilized with phosphoric acid.G35-A high molecular weight compound of a polyethylene glycol and a diepoxide that is esterified with nitroterephthalic acid.G36-1% Vinyl-5% phenylmethylpolysiloxane.G37-Polyimide.G38-Phase G1 containing a small percentage of a tailing inhibitor.19G39-Polyethylene glycol(av.mol.wt.about 1500).G40-Ethylene glycol adipate.G41-Phenylmethyldimethylsilicone(10% phenyl-substituted).G42-35% phenyl-65% dimethylpolysiloxane(percentages refer to molar substitution).G43-6% cyanopropyhenyl-94%dimethylpolysiloxane(percentages refer to molar substitution).G44-2% low molecular weight petrolatum hydrocarbon grease and 1% solution of potassium hydroxide.G45-Divinylbenzene-ethylene glycol-dimethylacrylate.G46-14% Cyanopropylphenyl-86% methylpolysiloxane.G47-Polvethylene glycol(av.mol.wt.of about 8000).G48-Highly polar,partially cross-linked cyanopolysiloxane.G49-Proprietary derivatized phenyl groups on a polysiloxane backbone.20SupportsNOTE-Unless otherwise specified,mesh sizes of 80 to 100or,alternatively,100 to 120 are intended.S1A-Siliceous earth for gas chromatography has been fluxcalcined by mixing diatomite with Na2CO3 flux and calcining above 900°.The siliceous earth is acid-washed ,then water-washed until neutral,but not base-washed.The silieous earth may be silanized by treating with an agent such as dimethyldichlorosilane21 to mask surface silanol groups.S1AB-The siliceous earth as described above is both acid-and base-washed.21S1C-A support prepared from crushed firebrick and calcined or burned with a clay binder above 900°with subsequent acid-wash.It may be silanized.S1NS-The siliceous earth is untreated.S2-Styrene-divinylbenzene copolymer having a nominal surface area of less than50㎡per g and an average pore diameter of 0.3 to 0.4 μm.S3-Copolymer of ethylvinylbenzene and divinylbenzene having a nominal surface ared of 500 to 600 ㎡per g and an average pore diameter of 0.0075μm.S4-Styrene-divinylbenzene copolymer with aromatic-O and –N groups, having a nominal surface area of 400 to 600㎡per g and an average pore diameter of 0.0076μm.S5-40-to60-mesh,high-molecular weight tetrafiuorethylenepolymer.S6-Styrene-divinylbenzene copolymer having a nominal surfasearea of 250 to 350㎡per g and an average pore diameter of 0.0091μm.S7-Graphitized carbon having a nominal surface area of 12㎡per g.S8-Copolymer of 4-vinyl-pyridine and styrene-divinylbenzene.S9-A porous polymer based on 2,6-diphenly-p-phenylene oxide. S10-A highly polar cross-linked copolymer of acrylonitrite and divinylbenzene.S11-Graphitized canbon having a nominal surface area of 100㎡per g modified with small amounts of petrolatum and polyethylene glycol compound,22S12-Graphitized carbon having a nominal surface ared of 100 ㎡per g.。
Review2-Hydroxypropyl-b -cyclodextrin (HP-b -CD):A toxicology reviewSarah Gould *,Robert C.ScottSafety Assessment,AstraZeneca UK Limited,Mereside,Alderley Park,Macclesfield,Cheshire SK104TG,United KingdomReceived 11November 2004;accepted 3March 2005Abstract2-hydroxylpropyl-b -cyclodextrin (HP-b -CD)is an alternative to a -,b -and c -cyclodextrin,with improved water solubility and may be more toxicologically benign.This paper reviews the toxicity of HP-b -CD,using both literature information and novel data,and presents new information.In addition,it includes a brief review from studies of the metabolism and pharmacokinetics of HP-b -CD in both humans and animals.This review concludes that HP-b -CD is well tolerated in the animal species tested (rats,mice and dogs),particularly when dosed orally,and shows only limited toxicity.In short duration studies,there were slight biochemical changes whereas studies of a longer duration,up to three months,produced additional minor haematological changes but no histopathological changes.When dosed intravenously,histopathological changes were seen in the lungs,liver and kidney but all findings were reversible and no effect levels were achieved.The carcinogenicity studies showed an increase in tumours in rats in the pancreas and intestines which are both con-sidered to be rat-specific.There were also non-carcinogenic changes noted in the urinary tract,but these changes were also reversible and did not impair renal function.There were no effects on embryo-foetal development in either rats or rabbits.HP-b -CD has been shown to be well tolerated in humans,with the main adverse event being diarrhoea and there have been no adverse events on kidney function,documented to date.Ó2005Elsevier Ltd.All rights reserved.Keywords:2-hydroxylpropyl-b -cyclodextrin;ToxicologyContents 1.Introduction ...........................................................................14522.Toxicology of HP-b -CD...................................................................14522.1.General toxicology .................................................................14522.1.1.Intraperitoneal administration ...................................................14522.1.2.Intravenous administration .....................................................14522.1.3.Acute intravenous studies ......................................................14522.1.4.Short-term studies............................................................14532.1.5.Chronic oral administration (via diet)..............................................14552.2.Genetic toxicity....................................................................14562.3.Carcinogenicity studies ..............................................................14562.4.Developmental toxicity ..............................................................14560278-6915/$-see front matter Ó2005Elsevier Ltd.All rights reserved.doi:10.1016/j.fct.2005.03.007*Corresponding author.Tel.:+441625512648;fax:+441625516809.E-mail address:sarah.gould@ (S.Gould)./locate/foodchemtoxFood and Chemical Toxicology 43(2005)1451–14593.Human toxicity profile (1456)4.Animal pharmacokinetic and metabolic profile (1456)5.Human pharmacokinetic and metabolic profile (1457)6.Discussion (1457)References (1458)1.IntroductionCyclodextrins(CDs)are useful formulation vehicles, which increase the amount of drug that can be solubi-lised in aqueous vehicles,thus increasing delivery of many useful medicinal agents to a biological system. Without a successful delivery system,many drugs could not be developed.Cyclodextrins are cyclic amylose-derived oligomers composed of a varying number of a-1-4-linked glucose units.These glucose chains form a cone-like cavity into which compounds may enter and form a water-soluble complex and thus change the drugÕs physical–chemical properties.The number of units determines the size of the cone-like cavity and its corresponding name(Szetjli, 1998;Uekama et al.,1998).For example,the most com-mon cyclodextrins used as formulation vehicles are a-, b-and c-cyclodextrin,with the corresponding number of glucose units(a=6,b=7,c=8).These cyclodextrin molecules,although similar in their unit make-up,pos-sess slightly different absorption rates,possibly due to differences in degradation processes(Antlsperger and Schmid,1996).a-,b-and c-cyclodextrins are all used successfully to incorporate drugs into aqueous vehicles(Antlsperger and Schmid,1996)and their toxicity profile has been studied extensively(WHO,1993;reviewed by Antlsper-ger and Schmid,1996).The toxicity profile of CDs can differ depending on the route of administration.For example,b-cyclodextrin administered orally,induces limited toxicity(Olivier et al.,1991;Bellringer et al., 1995)and in both rats and dogs is considered non-toxic at a daily dose of less than600mg/kg bw or3%and less in the diet(Fromming and Szejtli,1996).However,if b-cyclodextrin is administered at higher doses in animals via a subcutaneous route,it will cause a decrease in body weight gain,a decrease in liver weight,and nephrotoxi-city,with an increase in kidney weight,proximal tubular nephrosis and cellular vacuolation(Perrin et al.,1978; Fromming and Szejtli,1996).Parenteral administration also induces similar changes to the kidney proximal tu-bules(Frank et al.,1976).2-hydroxylpropyl-b-cyclodextrin(HP-b-CD),a hydroxyalkyl derivative,is an alternative to a-,b-and c-cyclodextrin,with improved water solubility proper-ties(Uekama et al.,1998;Yoshida et al.,1988)and may be slightly more toxicologically benign.This paper reviews the toxicity of HP-b-CD,using literature information together with novel in-house study data generated within AstraZeneca using HP-b-CD to assess the toxicology of a potential new drug with low water solubility.These new data add to the literature information regarding HP-b-CD and reduce the need for further assessments of this component in novel vehicles.A brief review of the metabolism and pharmacokinetics of HP-b-CD,with variants on the percentage administered and the route of administra-tion is also included,as well as a limited review of hu-man safety.2.Toxicology of HP-b-CDA number of toxicity studies have been conducted with HP-b-CD by either oral or intravenous administra-tion in a variety of species including mice,rats,monkeys and dogs for up to a period of12months.In addition, carcinogenicity,genetic toxicology and developmental toxicity studies have also been done.AstraZeneca (AZ)completed a number of additional studies in rats and dogs up to a period of1month dosing.These stud-ies are reviewed and summarized below(Table1sum-marizes data from AZ;Table2summarizes previously published data).2.1.General toxicology2.1.1.Intraperitoneal administrationIn mice,up to10,000mg/kg bw HP-b-CD has been administered acutely by intraperitonal(i.p.)injection and was neither lethal nor did it produce any toxicity (Fromming and Szejtli,1996).2.1.2.Intravenous administrationThe intravenous administration of HP-b-CD has been studied in mice,monkeys,rats and dogs after single or repeated doses for up to90days.2.1.3.Acute intravenous studiesIn the Cynomolgus monkey,a single intravenous dose of10,000mg/kg of50%w/v HP-b-CD was not lethal (Brewster et al.,1990).In mice,a single intravenous dose of up to2000mg/kg bw was also not lethal(Fromming and Szejtli,1996).1452S.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–1459In AZ acute studies(see Table1)in the rat(Alpk: AP f SD;Wistar derived rat),a single intravenous dose of2250mg/kg of45%w/v HP-b-CD was not tolerated: there were premature deaths and adverse clinical signs, including decreased activity,breathing irregularities and the animals were cold to touch(TLR3056).How-ever,when the HP-b-CD was reduced to1000mg/kg of20%w/v HP-b-CD,in a repeat study,there were no premature deaths and no adverse clinical signs.These acute studies included measurement of body weight and food consumption,in-life clinical observations and macroscopic examinations.2.1.4.Short-term studiesAZ has completed a number of short-term studies (Table1)in which several parameters were measured to determine overt toxicity including body weight and food consumption,in-life observations,haematology(haemo-globin,haematocrit,total red blood cell count,mean corpuscular haemoglobin,mean corpuscular haemoglo-bin concentration,mean corpuscular volume,platelet count,total leukocyte count,lymphocytes,monocytes, neutrophils,basophils)and clinical chemistry parameters (glucose,urea,creatinine,total protein,albumin,total bilirubin,alkaline phosphatase,alanine transferase, aspartate transferase,sodium,potassium,cholesterol, glutamate dehydrogenase,triglycerides),bone marrow smear,organ weights(liver,kidney),microscopic exami-nation of pelvic,thoracic and abdominal cavities and macroscopic examinations of the following tissues: adrenal glands,bone,brain,epididymides,eyes,heart, intestines,kidneys,liver,lung,pancreas,lymph nodes, pituitary gland,prostate,salivary gland,skeletal muscle, skin,spinal cord,spleen,stomach,testes,thymus,thy-roid,tracheas,thyroid gland,tongue,urinary bladder, uterus,vagina,and any gross lesions noted in macro-scopic examination.2.1.4.1.Intravenous administration.Alpk:AP f SD(Wis-tar-derived)rats were continuously infused for either4–7days with225mg/kg/day of11.25%w/v HP-b-CD via the femoral vein.Histopathology changes included foa-my macrophage infiltration of the lung,with some asso-ciated alveolitis haemorrhage,and atelectasis.In addition,renal cortical tubular vacuolation of the prox-imal convoluted tubules and mild reduced splenic extra-medullary haematopoiesis were also noted.Similar histopathology changes were also recorded in rats dosed with15%HP-b-CD and2.5%dextrose for4days (Table1).In a7-day rat study(Alpk:AP f SD;Wistar-derived) 2400mg/kg/day of5%w/v HP-b-CD was administered by continuous intravenous infusion.In this study, there was a reduction in water consumption,reduced plasma cholesterol and minor changes in the kidney, which included an increase in relative kidney weightTable1Summary of general toxicity studies with HP-b-CD(previously unpublished date from studies conducted by AstraZeneca)Route Studyduration(days)Species Animalnos.Dose(%)mg/kg/dayDatai.v.1Rat52250(45%)Premature deaths,adverse clinical signs:decreased activity,breathing irregularities1Rat51000(20%)No premature deaths,no adverse clinical signs7Rat10225(11.3%)Histopathology:foamy macrophage infiltration,alveolitis haemorrhage,actalctasis,renal cortical tubular vacuolation,of proximal tubs,mild reduced splenic extramedullaryhaematopoiesis4Rat3225(11.3%)Histopathology:foamy macrophage infiltration,alveolitis haemorrhage,actalctasis,renal cortical tubular vacuolation of proximal tubs,mild reduced splenicextramedullary haematopoiesis4Rat37200(15%)Histopathology:foamy macrophage infiltration,alveolitis haemorrhage,actalctasis,renal cortical tubular vacuolation of proximal tubules,mild reduced splenicextramedullary haematopoiesis7Rat32400(5%)Reduction in water consumption,reduced plasma cholesterol,increase in relativekidney weight,moderate renal cortical tubular vacuolationOral7Rat104500(45%)Increases in ALT,AST,GLDH7Rat10450(45%)NOEL2250(45%)Loose faeces,clinical pathology(increases in AST,ALT)4500(45%)Loose faeces,clinical pathology(increases in AST,ALT)28Rat20450(45%)Increase ALT4500(45%)Increase in water consumption,Loose faeces,increases in lymphocytes,reduction inreticulocyte,HCT,increase in platelet count,increases in ALP,ALT,AST,reductionsin creatinine,triglycerides,reduction in glucose concentration MTD/14Dog6540(45%)NOEL.No toxicological effects28Dog62250(45%)NOEL.No toxicological effectsNOEL:no observed effect level;ALT:amino transferase;ALP:amino phosphatase;AST:aspartame transferase;MTD:maximum tolerated dose.S.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–14591453(compared with body weight)and moderate renal cortical tubular vacuolation.In addition,mild foamy alveolar macrophages in the lung were also detected (Table1).In a14-day subacute and90-day subchronic intrave-nous toxicity studies,200mg/kg of20%w/v HP-b-CD was administered to Sprague Dawley rats and Cynomol-gus monkeys on alternate days(doses were given every second day).In these studies,there were no toxicologi-cally adverse effects on the following parameters:body weight,body weight gain,food consumption,haematol-ogy,clinical chemistry,organ weights(cf brain or body weights)and macroscopic or microscopic histopathol-ogy(including kidney)(Brewster et al.,1990).Two,three month intravenous dosing studies were conducted in rat and dog,and for both studies,HP-b-CD was administered at doses of50,100or400mg/ kg/day.In the rat,there were no adversefindings at 50mg/kg/day.At100mg/kg/day,there were minimal histological changes in the urinary bladder(swollen epi-thelial cells),swollen and granular kidney tubular cells and an increase in Kupffer cells in the liver.At 400mg/kg/day,there was a decrease in body weight and food consumption,increase in water consumption, a decrease in haematocrit,haemoglobin and erythro-cytes,and an increase in creatinine,bilirubin and aspar-tate and alanine aminotransferase plasma levels(AST and ALT,respectively).The weight of the spleen,adre-nals and kidneys increased and histopathological changes included foamy cells in the lungs,increased spleen red pulp hyperplasia,increased rough endoplas-mic RES aggregates in the liver and increased round cells in the Kieran space of the liver.Following a one month recovery period,most of the toxicological changes had reversed.However,there were still small elevations in AST and ALT levels and only a partial reversal of the urinary tract bladder and lung changes. In the dog,there were no adverse effects at50or 100mg/kg/day.At400mg/kg/day,there were slight in-creases in ALT and AST and total bilirubin.Histologi-cal changes were seen in the lung(foamy cell)and there were swollen epithelial cells of the urinary bladder and renal pelvis.At the end of the recovery period,the tox-icological changes had completely reversed except for an incomplete recovery of the swollen renal pelvis epithe-lium(Coussement et al.,1990).Table2Data from toxicology studies conducted with HP-b-CD(external publications)Route Study duration Species Animal noÕs Dose mg/kg/day Data Sourcei.v.1Monkey410,000No deaths Brewster et al.,19901Mouse Unknown2000No deaths Fromming and Szejtli,1996 14/90Rat10200No toxicological effects Brewster et al.,199014/90day Rat Unknown100Swollen epithelial bladder cells,swollenand granular kidney tubular cells,increase in Liver Kupffer cellsCoussement et al.,199014/90day Rat Unknown400Reduced body weight,food consumption,increase water consumption,decrease haematology parameters,andclinical chemistry,increase inspleen,adrenals kidneys,foamy cellsin lungs,spleen hyperplasia,increased RES aggregates in the liverCoussement et al.,1990Monkey4200No significant toxicity Brewster et al.,1990 3month Rat Unknown50NOEL Coussement et al.,1990100Minimal histological change inthe bladder,kidney and liverDog Unknown100NOAEL Coussement et al.,1990400Slight increase in plasma liverenzymes and histopathology in lung,bladder and pelvisi.p.Acute Mouse Unknown1000No deaths Fromming and Szejtli,1996 Oral1year Rat100500NOEL Van Cauteren et al.,19972000Small reduction in body weight,minor haematology and clinicalchemistry changes(includingincreased plasma liver enzymes)and histology changes urinarytract,liver,pancreasDog Unknown1000NOEL Van Cauteren et al.,19972000Loose faeces,urinary tract histopathologyOEL:no observed effect level.1454S.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–14592.1.4.2.Oral administration.AZ has completed suba-cute and subchronic toxicity studies in rats and dogs, which assessed the toxicity of HP-b-CD.Animals were weighed and food consumption monitored,standard haematology(red blood cell count,haemoglobin,hae-matocrit,mean cell volume,mean cell haemoglobin, mean cell haemoglobin concentration,total white blood cell count,differential white blood cell count,platelet count,prothrombin time and activated partial thromb-oblastin time),clinical chemistry(glucose,urea,creati-nine,total protein,albumin,total bilirubin,alkaline phosphatase,alanine transferase,aspartate transferase, sodium,potassium,cholesterol,glutamate dehydroge-nase,triglycerides)and urinalysis were completed both pre-study and during compound administration.At ter-mination,organ weight measurements were taken(for 7-day studies only liver and kidney were taken)for1-month studies organs weights included:adrenal glands, brain,epididymides,heart,kidney,liver(minus gall bladder),lungs,ovaries,pituitary,prostate gland, spleen,testes,thyroid glands(with parathyroid)and uterus(with cervix).Macroscopic and microscopic examinations were completed(see2.1.4for details for 7-day studies;for one-month studies the following tis-sues were taken:abnormal tissues,adrenal glands,aorta (thoracic)bone(femur)bone marrow(sternum),brain (whole),bronchus,cervix,epididymides,eyes,eyelids, gall bladder,heart(portions from4chambers and pap-illary muscle)intestine-duodenum,intestine-colon, intestine-rectum,kidneys,lacrimal glands,liver(left lat-eral and right median lobes),lungs,lymph node-bron-chial,cervical,mesenteric,mammary gland(female), muscle(quadriceps),nerve(sciatic),oesophagus,ova-ries,pancreas,pituitary gland,prostate gland,salivary gland-parotid,salivary gland-sublingual,salivary gland-submaxillary,skin(lateral thigh),spinal cord (lumbar and cervical)spleen,stomach,fundic area, stomach pyloric area,testes,thymus gland,thyroid and parathyroid,tongue,trachea,urinary bladder, uterus and vagina.For the dog studies,electrocardio-gram and direct blood pressure measurements were also made at the start and end of the study period.Heart rate,P–R,QRS and Q–T intervals were derived from the ECG traces.In a7-day oral study in rats(Alpk:AP f SD;Wistar-de-rived)4500mg/kg/day45%w/v HP-b-CD caused changes in the plasma ALT,AST and glutamate dehy-drogenase(GLDH)levels.The changes were particu-larly apparent in the females,but these changes were not accompanied by any histopathological changes (Table1).In another rat study,AZ administered450,2250or 4500mg/kg/day45%w/v HP-b-CD to rats(Alp-k:AP f SD;Wistar-derived)for7days and4500mg/kg/ day45%w/v HP-b-CD for14days and showed that HP–CD was well tolerated at doses of up to4500mg/kg/day.There were no toxicologicalfindings at doses of450mg/kg/day and no treatment related effects seen on body weights,food or water consumption.For doses of2250mg/kg/day and above,loose faeces were seen from day2.Clinical pathology showed marked increases in plasma GLDH activity at both2250and4500mg/kg/ day and minor increases in plasma AST and ALT levels from day4in males dosed with4500mg/kg/day and day 8in females dosed with2250mg/kg/day.There were no necropsy or histological changes in the liver,including at the electronic microscopy level or in the kidney (Table1).In a one-month rat study,Alpk:AP f SD(Wistar-de-rived)rats were dosed orally with450and4500mg/kg/ day45%w/v HP-b-CD.Loose faeces were observed in animals dosed with4500mg/kg/day HP-b-CD from day13.All male rats dosed with HP-b-CD showed an increase in water consumption and both sexes at 4500mg/kg/day showed an increase in white blood cells (WBC)attributed to an increase in lymphocytes. Changes in red cell parameters were also detected, including a small reduction in reticulocytes and haema-tocrit,a slight increase in platelet count in males dosed with4500mg/kg/day and a decrease in haemoglobin (Hb)in males dosed with450mg/kg/day.Increases in plasma liver enzymes were detected in all animals dosed with HP-b-CD,including:increases in plasma ALP activity in males dosed with4500mg/kg/day;increases in plasma ALT activity in females dosed with450mg/ kg/day and in both sexes dosed with4500mg/kg/day; an increase in AST activity in both sexes dosed with 4500mg/kg/day and an increase in glutamate dehydro-genase(GLDH)activity in both sexes dosed with 4500mg/kg/day.There were slight reductions in plasma creatinine and triglycerides concentrations in males dosed with450and4500mg/kg/day HP-b-CD and a reduction in plasma glucose concentration was detected in females dosed with4500mg/kg/day(Table1).In the Beagle dog,AZ conducted a maximum toler-ated dose study for up to14days.The dose was with McIlvaines buffer containing a540mg/kg/day45%w/v solution of HP-b-CD and there were no toxicological ef-fects(Table1).AZ also conducted a one-month oral toxicity study in Beagle dogs with2250mg/kg/day 45%w/v HP-b-CD and found no toxicological effects (Table1).2.1.5.Chronic oral administration(via diet)Twelve-month oral toxicity studies in rat and dog were reported in the literature.In the12-month rat(Wistar)study,HP-b-CD was administered via the diet at doses500,2000and 5000mg/kg/day.At500mg/kg/day there were no toxi-cological effects.At2000mg/kg/day there was a small reduction in male body weight,increased serum chloride and liver plasma enzymes,reduced urinary pH andS.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–14591455volume in males,slight increase in pancreas weight and histopathology changes(swollen urinary tract epithelial cells,centrilobular swelling in the liver and focal hyper-plasia in the pancreas).At5000mg/kg/day,toxicity was more pronounced with,in addition to the above changes,an increase in female body weight,and increase in food and water consumption,increase in white blood cells,decrease in thrombocytes,decrease in lipids,occult urinary blood in females,increased pancreas,kidney and lung weights,and increases in foamy cells in the lung(Van Cauteren,personal presentation,1997).In the12month dog study,HP-b-CD was adminis-tered by oral gavage at dose levels of500,1000and 2000mg/kg/day.The no effect dose level was1000mg/ kg/day.The higher doses showed softened faeces and urinary tract histological changes.(Van Cauteren,per-sonal presentation,1997).2.2.Genetic toxicityThe available literature reports were limited in detail. In an Ames assay(up to1000l g/plate)and an in vivo micronucleus test(up to5000mg/kg/day;species un-known)there was no evidence that HP-b-CD was geno-toxic(Coussement et al.,1990).HP-b-CD has also been reported to be negative in an unscheduled DNA synthe-sis test(UDS)assay(for DNA damage),a mouse lym-phoma assay(for gene mutation)and in a human lymphocyte test(for chromosomal aberration)(Van Cauteren,personal presentation,1997).2.3.Carcinogenicity studiesThere are reports in the literature of thefindings from an18-month mouse(Swiss strain)and a2-year rat(Wis-tar Strain)carcinogenicity study,which both dosed HP-b-CD in the diet,at dose levels of500,2000and 5000mg/kg body weight/day.In the mouse study,there was no effect on survival and no increase in total tumour incidence of individ-ual tumour type and thus;the study concluded there was no evidence of primary carcinogenic potential in the mouse(Van Cauteren,personal presentation, 1997).In the2-year rat study,there was no effect on survival or increase in total tumour incidence at doses of up to 5000mg/kg body weight/day.However,there were some changes including:increases in polypoid tumours of the large intestine at the high dose(incidence0/100controls, 4/50males,2/50females),tumours of the exocrine pan-creas at all dose levels and changes in the urinary tract (swelling and vacuolation of renal cortical tubules,uro-thelium of pelvis and urinary bladder,enlargement of secondary lysosomesfilled with heterogeneous inclu-sions).Changes in the pancreas were initially seen at 12months,with exocrine pancreatic hyperplasia,which developed to exocrine pancreatic neoplasia by24 months.Changes in the urinary tract were also seen by light microscope as swelling and vacuolation in cells of the renal cortical cells,urothelium of pelvis and urinary bladder.When examined by electron microscopy,there was evidence of enlarged secondary lysosomesfilled with heterogeneous inclusions(Van Cauteren,personal pre-sentation,1997).2.4.Developmental toxicityDevelopmental toxicity studies,in rats and rabbits using either oral or intravenous administration are re-ported in the literature.In an intravenous embryo-foetal development study in rats(dosing day6–16of pregnancy),400mg/kg/day caused slight maternal toxicity,but there were no ad-verse effects observed in the offspring.In a similar study in rabbits(dosing day6–18of pregnancy),there were no adverse effects at doses of up to400mg/kg/day(Couss-ement et al.,1990).In an oral teratogenic and embryotoxicity study in rats(dosing day6–16of pregnancy),maternal toxicity, embryotoxicity and teratogenicity were not present at doses of up to400mg/kg/day.In an oral study in rabbits (dosing day6–18of pregnancy),slight maternal toxicity and embryotoxicity was present at1000mg/kg(Cousse-ment et al.,1990).3.Human toxicity profileA number of clinical studies are reported in the liter-ature and have shown that HP-b-CD was well tolerated and safe in the majority of patients receiving HP-b-CD at daily oral doses of4–8g for at least2weeks(Irie and Uekama,1997).Higher oral daily doses of16–24g when given for14days to volunteers,resulted in increased incidences of soft stools and diarrhoea.There-fore,based on these clinical data,HP-b-CD was consid-ered to be non-toxic(at least for14days)if the daily dose is<16g.In an intravenous dosing study(Seiller et al.,1990) single doses up to3g were found to have no measurable effect on kidney function and were well-tolerated by all volunteers.Following a1week intravenous study at a single dose level of1g,no adverse effects were reported (Janssen Technical Bulletin,1992).4.Animal pharmacokinetic and metabolic profileThe pharmacokinetics and metabolism of HP-b-CD have been examined in rats and dogs following single and repeat intravenous(Monbaliu et al.,1990)and oral administration(Monbaliu et al.,1990;Gerloczy et al., 1990).1456S.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–1459After a single200mg/kg intravenous dose in rats and dogs,14C-HP-b-CD was eliminated rapidly(more than 90%in4h),almost completely as the intact compound and mostly by renal excretion.The excretion in faeces and expired air was minimal.The plasma elimination half-life was0.4h in rats and0.8h in dogs.After oral administration of HP-b-CD in both rats and dogs, 86%was excreted via the faeces in both species,where as less than5%was excreted in the urine.In rats,about 10%of the administered dose was found in the expired air.The absolute bioavailability was calculated from this study and estimated at3.3%in the dog and less in the rat.In both rats and dogs following intravenous admin-istration,tissue distribution was limited:in rats the high-est concentration was found in the kidney and lung and in dogs,the highest concentrations were in the kidney and the liver.The concentration of HP-b-CD was higher in the renal cortex than the medulla.A3-month intrave-nous toxicity study was conducted in both rat and dog at doses of50,100or400mg/kg/day HP-b-CD and showed that plasma concentrations increased linearly with dose in both species(Monbaliu et al.,1990).A separate oral study in rats was also conducted by Gerloczy et al.(1990)and showed that up to6%of a sin-gle oral dose was absorbed from the gastrointestinal tract within5min.Faecal excretion was found to be the main route of elimination.Of the dose administered, approximately3%was eliminated by the kidney in the urine and70%in the faeces within72h.Thisfinding concurs with Monbaliu et al.(1990)data following oral administration.Analysis of exhaled air showed that3% of the total dose was metabolised.Tissue distribution analysis showed that the largest amounts of HP-b-CD were detected in the liver(3–5%of the dose)and kidneys (0.2–0.35%)(Gerloczy et al.,1990),suggesting that there may be a slight difference in distribution depending upon the route of administration;Monbaliu et al. (1990)had shown the kidney as the major organ con-taining HP-b-CD following intravenous administration.The steady state volume of distribution(Vdss)for HP-b-CD in rats(Monbaliu et al.,1990),dogs(Monba-liu et al.,1990)and humans(Messens et al.,1991)corre-sponds well with the extracellularfluid volume of each species suggesting that there are no deep compartments or storage in pools.The total plasma clearances(CLt)in all species tested are similar to that of inulin and clear-ance through the kidneys is independent of dose admin-istered and nearly equivalent to the glomerularfiltration rate thus,elimination is dependent on renal function (Frijlink et al.,1991).5.Human pharmacokinetic and metabolic profileThe pharmacokinetics of HP-b-CD have been studied in healthy volunteers after single intravenous and oral dosing(Szathmary et al.,1990).Following intravenous dosing at0.5,1.0,1.5.2.0,2.5or3.0g,plasma levels of unchanged HP-b-CD declined rapidly and showed a bi-phasic decline.There were no differences between males and females and dose proportionality was demon-strated.Pharmacokinetic parameters such as half life, clearance and Vdss were shown to be independent of dose and urine levels suggested that elimination was al-most totally via the kidneys with no sign of tubular reabsorption.It was postulated that any remaining drug might be eliminated by other pathways,most likely by metabolism.Following oral administration,HP–CD could not be detected in either the plasma after1h or urine indicating that there was no absorption from the gastrointestinal tract and that oral bioavailability in humans was low(Szathmary et al.,1990).6.DiscussionThe available literature shows that the toxicity of HP-b-CD in animals has been extensively studied.HP-b-CD is well tolerated in most species,particularly if dosed or-ally and shows limited toxicity,depending upon dose and route of administration.HP-b-CD is also well toler-ated in humans,with the main adverse effect being diar-rhoea with no effects,documented to date,on kidney function.In the animal studies,when administered orally,HP-b-CD,at either high single doses or a prolonged period of administration,induces minor biochemical changes. For example,after7days administration in the rat, 450mg/kg/day was found to be a no effect dose level and450and4500mg/kg of45%HP-b-CD when admin-istered for one month produced only minor haematol-ogy changes and increases in plasma liver enzyme levels.There was no evidence of any histopathological changes,even at the highest oral dose,demonstrating that HP-b-CD was well tolerated and toxicologically parison of effects in different species shows that the dog may be slightly less sensitive to the effects of HP-b-CD than the rat.For example,in the one month dog study,an oral a dose of2250mg/kg/day 45%HP-b-CD was a no effect dose,whereas in the rat,only7days administration at this dose caused minor increases in AST and ALT.Chronic oral dosing in the rat produced limited toxicity,with onlyfindings at the highest doses of either5000mg/kg/day or2000mg/kg/ day;however,in this chronic study the HP-b-CD was administered via diet,which may have reduced the bio-availability and maximum systemic exposure compared with oral gavage dosing.Administration of HP-b-CD via intravenous infusion induced some minor clinical observations,as well as biochemical and histopathology changes.The target organs were the lungs,where there was an increase inS.Gould,R.C.Scott/Food and Chemical Toxicology43(2005)1451–14591457。