Melt flow characteristics in gas-assisted laser cutting
- 格式:pdf
- 大小:132.33 KB
- 文档页数:7

炼钢转炉顶吹氧气射流特性的CFD数值分析李强;李明明;李琳;邹宗树【摘要】联合标准的k~ε湍流模型,建立了转炉顶吹可压缩氧气射流的CFD模型.对氧气在拉瓦尔喷管内外的射流行为进行了数值模拟研究,考察了不同操作压力以及环境温度下氧射流的流动行为,并分析了射流激波现象.实验结果表明,当操作压力小于设计压力时,喷管出口处形成斜激波,压缩波与膨胀波交替进行;当操作压力大于设计压力时,喷管出口形成扇形膨胀波,膨胀波与压缩波交替进行.模拟结果也表明,环境温度增加,射流动压基本不变,但超音速区长度增加.通过回归分析,给出了射流核心长度与操作压力的定量关系.【期刊名称】《东北大学学报(自然科学版)》【年(卷),期】2013(034)006【总页数】4页(P828-831)【关键词】氧气射流;拉瓦尔喷管;射流核心长度;转炉;数值模拟【作者】李强;李明明;李琳;邹宗树【作者单位】东北大学材料与冶金学院,辽宁沈阳110819;东北大学材料与冶金学院,辽宁沈阳110819;东北大学材料与冶金学院,辽宁沈阳110819;东北大学材料与冶金学院,辽宁沈阳110819【正文语种】中文【中图分类】TP274顶吹及复吹转炉生产过程中,氧气通过氧枪将压力能转化为动能,形成超音速射流.氧气射流的特性对于氧枪枪位制度的制定、化渣、脱碳、脱磷及熔池的搅拌特性都至关重要.对自由射流行为,有学者[1-3]进行了冷模型及热模型的实验研究,然而对于预测实际转炉内高马赫数、高温等复杂条件下射流动力学行为还比较困难.近年来随着CFD技术的发展,Hatta等[4]和Peng等[5]通过建立数学模型,模拟了稳态、准一维条件下气体在拉瓦尔喷管内的流动特性.Odenthal等[6]对单孔射流流经拉瓦尔喷管的稳态、可压缩行为进行了数值模拟.Wang等[7]研究了转炉内多孔可压缩气体射流在转炉内的流动特性,并考虑了流体密度和黏性、温度以及马赫数对气体流动特性的影响.Odenthal等[8]研究了不同操作工况时氧气在拉瓦尔喷管内外的流动,并给出了射流在喷管外超音速区长度与环境压力的关系.这些研究成果能够预测氧射流在转炉内的流体动力学行为,然而,对于非设计工况下易于出现激波现象的研究还少见报道.本文建立了二维轴对称、可压缩、稳态条件下氧气在转炉氧枪拉瓦尔喷管内外流动的数学模型,湍流模型选择标准的k~ε湍流模型,考察了设计工况条件下、非设计操作工况下和环境温度下自由射流运动行为,并分析了射流激波现象,同时给出射流超音速区即射流核心长度与操作压力、环境温度的相互关系,为指导转炉氧枪吹炼操作提供理论依据.1 数学模型1.1 控制方程在大多数转炉生产过程中,氧枪射流马赫数达到2.0左右,因此气体可压缩性对气流的衰减规律影响很大,模型中气体射流作为可压缩气体处理.建立描述气体自由射流数学模型:连续性方程·(ρu)=0.(1)动量方程·(ρuu)=-p+μeff2u.(2)能量方程(3)理想气体状态方程(4)式中:ρ为气体密度,kg/m3;u为速度矢量,m/s;μeff为有效湍流黏度,kg/(m·s);T为气体温度,K;κeff为有效导热系数,W/(m·K);R为气体常数;M为摩尔质量,kg/mol;τij为黏性应力量,湍流模型选择标准的k~ε模型,其控制方程为Gk-ρε-Ym,(5)(6)上述湍流模型参数计算过程中均取标准值.1.2 模型几何参数及边界条件拉瓦尔喷管尺寸:入口直径36 mm,喉口直径30 mm,出口直径43.4 mm,收缩段长度54 mm,喉口段长度12 mm,扩张段长度96 mm,设计马赫数2.25.入口设为压力入口,出口及压力边界均设为压力出口,采用无滑移壁面边界条件,近壁处采用标准壁函数.CFD计算采用基于可压缩密度方法,离散格式采用二阶迎风格式.2 结果及讨论2.1 设计工况下射流特性根据某厂操作实践,滞止温度T0为308 K,炉内压力为101 325 Pa.根据等熵理论,设计工况时操作压力p0为158 067 Pa,出口马赫数Ma为2.25.数值模拟结果如图1和图2所示.图1 设计工况下射流特性Fig.1 Characteristic of jet under designcondition(a)—速度场; (b)—温度场.图2 设计工况下射流中心线上速度与温度变化Fig.2 Relationship of velocity and temperature oncentre line of jet under design condition为了验证数值模拟的正确性,模拟结果与等熵理论计算值进行了比较,结果见表1.实验模拟结果与理论计算结果的质量流量误差为1.1%.同时由图1和图2可知,喷管出口外射流Ma,T,p及ρ存在振荡,即存在压缩波和膨胀波.表1 CFD模拟结果与等熵理论计算值比较Table 1 Comparison between CFD simulation results and isentropic theory solution类别p/p0T/T0ρ/ρ0MaG/(kg·s-1)Δm/m/%CFD喉口0.54950.84440.65100.971.9822出口0.09200.50940.18032.21等熵理论喉口0.52830.83330.63391.002.0036出口0.08650.49890.17402.251.12.2 非工况条件下的射流特性对于实际转炉操作,由于操作水平和设备误差,一般在非设计工况下进行.当喷枪处于设计工况条件喷吹时,出口外存在压缩波即斜激波的临界点,此时射流为完全膨胀射流.进口压力低于设计工况压力时,则出口外侧为压缩波强波,此时射流为过度膨胀射流,射流中压缩波与膨胀波交替进行;进口压力高于设计工况时,则出口外侧为膨胀波,射流为未完全膨胀射流,射流中膨胀波与压缩波交替进行.图3给出了不同操作压力下射流特征,其中a~d的操作压力逐渐增大.图3 操作压力对射流特性的影响Fig.3 Influence of operating pressureon characteristic of jet当操作压力小于设计工况压力时(图3a),出口处形成斜激波,射流有效截面减小,压缩波与膨胀波交替进行.由图3c~3d可知,当操作压力大于设计工况压力时,射流在出口外膨胀,形成扇形的膨胀波,膨胀波与压缩波交替进行.当压力进一步增加时(图3d),射流与环境间较大的压力差形成马赫盘.氧气流股的收缩和膨胀,使得射流很不稳定,且能量损失较大,不利于吹炼,因此实际生产中应避免压缩波和膨胀波的产生.为了考察操作压力与射流特性的定量关系,以无因次射流核心长度x/de和无因次操作压力p/p0作图,如图4所示.图4 射流核心长度与操作压力关系Fig.4 Relationship between length of jet supersonicregion and operating pressure由图4可知,无因次射流核心长度与无因次操作压力有很好的线性关系.经线性回归所得定量关系为:x/de=12.93p/p0+10.3.所得实验结果与Naito等[2]和Tago 等[3]的研究结果较吻合.2.3 环境温度对射流特性影响在转炉炼钢顶吹及复吹过程中,随吹炼过程的进行,转炉内温度逐渐增加,炉内温度达1 600 K以上,在整个一炉钢的吹炼过程中,熔池温度约提高350 ℃左右.低温氧射流从喷口射出通过热的环境,到达熔池表面之前被加热.由于密度等气体性质的剧烈变化,气体加热过程对射流的行为有很大影响.然而很少有环境温度对气体行为影响的研究,因为包括气体之类的射流行为的精确测量非常困难[9].图5和图6给出了设计工况下环境温度为 300,1 773 K 时射流特性的比较.结果表明,环境温度影响射流的膨胀,环境温度增加,喷管出口外射流势流核心区的速度基本不变,但射流超音速区长度增加.这是由于环境温度影响气体的密度,温度越高,气体密度越小,导致气体射流速度衰减越慢,此实验结果与文献[3]一致.图6也表明,在亚音速区,射流速度逐渐趋于一致.图5 不同环境温度下射流特性Fig.5 Characteristic of jet under differentambient temperatures(a)—1 773 K; (b)—300 K.图6 不同温度下射流中心线上速度曲线Fig.6 Velocity curves of jet on center lineunder different temperatures3 结论1) 数值模拟结果与等熵理论计算结果相一致,设计工况下,喷管出口外形成激波.2) 当入口压力小于操作压力时,出口处形成斜激波,射流有效截面减小,压缩波与膨胀波交替进行;当入口压力大于操作压力时,射流在出口外膨胀,形成扇形的膨胀波,膨胀波与压缩波交替进行,当压力进一步增加时,形成马赫盘;给出了无因次射流核心长度与无因次入口压力之间的定量关系.3) 环境温度对射流特性有较大影响,环境温度增加,核心区长度增加,但势流核心速度基本不变.通常氧枪设计过程中都要经过冷态实验,但冷态实验的结果也需要根据转炉内温度特点进行相应的校正.参考文献:[1] Higuchi Y,Tago Y.Effect of lance design on jet behavior and spitting rate in top blown process[J].ISIJ International,2001,41(12):1454-1459.[2] Naito K,Ogawa Y,Inomoto T,et al.Characteristics of jets from top-blown lance in converter[J].ISIJ International,2000,40(1):23-30.[3] Tago Y,Higuchi Y.Fluid flow analysis of jets from nozzles in top blown process[J].ISIJ International,2003,43(2):209-215.[4] Hatta N,Fujimoto H,Ishii R,et al.Numerical study on supersonic flows of gas-liquid particle mixture in a de Laval nozzle[J].ISIJ International,1989,29(11):911-918.[5] Peng Y,Han T.Gas-particle flow in a de Laval nozzle with curved convergent configuration[J].ISIJ International,1996,36(3):263-268. [6] Odenthal H J,Kempken J,Schlüter J,et al.Advantageous numerical simulation of the converter blowing process[J].Iron Steel Technology,2007,4(11):71-89.[7] Wang W J,Yuan Z F,Matsuura H.Three-dimensional compressibleflow simulation of top-blown multiple jets in converter[J].ISIJ International,2010,50(4):491-450.[8] Odenthal H J,Falkenreck U,Schluter J.CFD simulation of multiphase melt flows in steelmaking convertor[C]//European Conference on Computational Fluid Dynamics.Delft:TU Delft,2006.[9] Sumii I,Kishimoto Y,Kikuchi Y,et al.Effect of high-temperature field on supersonic oxygen jet behavior[J].ISIJ International,2006,46(9):1312-1317.。

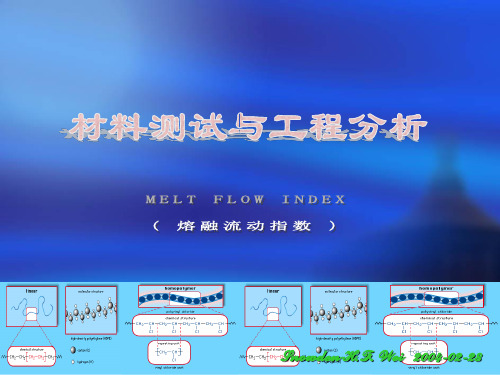
Designation:D1238–04Standard Test Method forMelt Flow Rates of Thermoplastics by Extrusion Plastometer1This standard is issued under thefixed designation D1238;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon(e)indicates an editorial change since the last revision or reapproval.1.Scope*1.1This test method covers measurement of the rate of extrusion of molten resins through a die of a specified length and diameter under prescribed conditions of temperature,load, and piston position in the barrel as the timed measurement is being made.1.2Procedure A is a manual cutoff operation based on time used for materials havingflow rates that fall generally between 0.15and50g/10min.Procedure B is an automatically timed flow rate measurement used for materials havingflows from 0.50to900g/10min.By both procedures,the piston travel is generally the same during the timed measurement;the piston foot is about46and20.6mm above the parableflow rates have been obtained by these procedures in interlaboratory round-robin measurements of several materials described in 13.1.Provision is made for calculation of melt volume-flow rate as well as melt mass-flow rate.N OTE1—Round-robin testing indicates this test method may be suit-able atflow rates up to1500g/10min if the timing clock resolves the elapsed time to the nearest0.01s.N OTE2—This test method and ISO1133-1991are technically equiva-lent.1.3This standard does not purport to address the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.Specific precautionary statements are given in5.7,10.2.12,and14.1.2.2.Referenced Documents2.1ASTM Standards:2D618Practice for Conditioning Plastics for TestingD883Terminology Relating to PlasticsE691Practice for Conducting an Interlaboratory Study toDetermine the Precision of a Test Method2.2ANSI Standard:B46.1on Surface Texture32.3ISO Standard:ISO1133-1991Determination of the Melt-Mass Flow Rate (MFR)and the Melt V olume-Flow Rate(MVR)of Ther-moplastics33.Terminology3.1General:3.1.1For definition of some of the technical terms used in this test method refer to Terminology D883.4.Significance and Use4.1This test method is particularly useful for quality control tests on thermoplastics.N OTE3—Polymers havingflow rates less than0.15or greater than900 g/10min may be tested by the procedures in this test method;however, precision data have not been developed.4.2This test method serves to indicate the uniformity of the flow rate of the polymer as made by an individual process and, in this case,may be indicative of uniformity of other proper-ties.However,uniformity offlow rate among various polymers as made by various processes does not,in the absence of other tests,indicate uniformity of other properties.4.3Theflow rate obtained with the extrusion plastometer is not a fundamental polymer property.It is an empirically defined parameter critically influenced by the physical proper-ties and molecular structure of the polymer and the conditions of measurement.The rheological characteristics of polymer melts depend on a number of variables.Since the values of these variables occurring in this test may differ substantially from those in large-scale processes,test results may not correlate directly with processing behavior.4.4Theflow rate of a material may be measured under any of the conditions listed for it in8.2.Additional characterization of a material can be obtained if more than one condition is used.In case two conditions are employed,a Flow Rate Ratio (FRR)may be obtained by dividing theflow rate at one condition by theflow rate at the other condition.1This test method is under the jurisdiction of ASTM Committee D20on Plastics and is the direct responsibility of Subcommittee D20.30on Thermal Properties (Section D20.30.08).Current edition approved March1,2004.Published April2004.Originally approved st previous edition approved in2001as D1238-01e1.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTMStandards volume information,refer to the standard’s Document Summary page on the ASTM website.3Available from American National Standards Institute(ANSI),25W.43rd St., 4th Floor,New York,NY10036.1*A Summary of Changes section appears at the end of this standard. Copyright©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA19428-2959,United States.5.Apparatus5.1Plastometer :5.1.1The apparatus shall be a dead-weight piston plastom-eter consisting of a thermostatically controlled heated steel cylinder with a die at the lower end and a weighted piston operating within the cylinder.The essential features of the plastometer,illustrated in Figs.1and 2,are described in 5.2-5.8.All dimensional measurements shall be made when the article being measured is at 2365°C.5.1.2Relatively minor changes in the design and arrange-ment of the component parts have been shown to cause differences in results among laboratories.It is important,therefore,for the best interlaboratory agreement that the design adhere closely to the description herein;otherwise,it should be determined that modifications do not influence the results.5.2Cylinder —The steel cylinder shall be 50.8mm in diameter,162mm in length with a smooth,straight hole 9.550460.0076mm in diameter,displaced 4.8mm from the cylinder axis.Wells for a thermal sensor (thermoregulator,thermistor,etc.)and thermometer shall be provided as shown in Fig.1.A 3.2-mm plate shall be attached to the bottom of the cylinder to retain the die.A hole in this plate,centered under the die and countersunk from below,allows free passage of the extrudate.The cylinder may be supported by at least two6.4-mm high-strength screws at the top (radially positioned at right angles to the applied load)or by at least two 10-mm diameter rods screwed into the side of the cylinder for attaching to a vertical support.The essential dimensions of a satisfactory cylinder of this type are shown in Fig.1(Note 4).The cylinder bore should be finished by techniques known to produce approximately 12rms or better in accordance with ANSI B46.1.N OTE 4—Cylinders made of SAE 52100or other equivalent steel heat-hardened to 60–65Rockwell Hardness Scale C give good service when used at temperatures below 200°C.Cylinder liners of cobalt-chromium-tungsten alloy are also satisfactory to 300°C.5.3Die —The outside of the steel die shall be such diameter that it will fall freely to the bottom of the 9.550460.0076mm diameter hole in the cylinder (Note 5).The die shall have a smooth straight bore 2.095560.0051mm in diameter and shall be 8.00060.025mm in length.The bore and its finish are critical.It shall have no visible drill or other tool marks and no detectable eccentricity.The die bore shall be finished by techniques known to produce approximately 12rms or better in accordance with ANSI B46.1.N OTE 5—Recommended die material is tungsten carbide.Also satisfac-tory are steel,synthetic sapphire,and cobalt-chromium-tungsten alloy.5.4Piston :5.4.1The piston shall be made of steel with an insulating bushing at the top as a barrier to heat transfer from the piston to the weight.The land of the piston shall be 9.474260.0076mm in diameter and6.3560.13mm in length.ThepistonFIG.1General Arrangement of ExtrusionPlastometerFIG.2Details of ExtrusionPlastometerdesign may incorporate means for land replacement,for example,having threads and flats immediately above the land.Above the land,the piston shall be no larger than 8.915mm in diameter (Note 6).The finish of the piston foot shall be 12rms in accordance with ANSI B46.1.If wear or corrosion is a problem,the piston should be of stainless steel and equipped with a detachable foot for ease of replacement.N OTE 6—To improve standardization it is preferable that the piston be guided with a loose-fitting metal sleeve at the top of the cylinder.N OTE 7—Pistons of SAE 52100steel with the bottom 25mm,including the foot,hardened to a Rockwell hardness,C scale,of 55to 59have been found to give good service when used at temperatures below 200°C.5.4.2The piston shall be scribed with two reference marks 4mm apart in such fashion that when the lower mark coincides with the top of the cylinder or other suitable reference point,the bottom of the piston is 48mm above the top of the die (see Fig.1).5.4.3The combined weight of piston and load shall be within a tolerance of 60.5%of the selected load.5.5Heater :5.5.1The equipment must have a heater capable of heating the apparatus so that the temperature at 10mm above the die can be maintained within 60.2°C of the desired temperature during the test.The temperature of the barrel,from 10mm to 75mm above the top of the die,must be maintained within 61%of the set temperature (°C).N OTE 8—At temperatures higher than 200°C this degree of temperature control may be more difficult to obtain.5.5.2Calibrate the temperature-indicating device by means of a light-gage probe-type thermocouple or a platinum-resistance temperature sensor having a short sensing length.4The thermocouple should be encased in a metallic sheath having a diameter of approximately 1.6mm with its hot junction grounded to the end of the sheath.Insert the tempera-ture sensor into the melt from the top of the cylinder so that it is 1061mm above the upper face of the die.The temperature sensors shall be used with a potentiometer having a sensitivity of at least 0.005mV ,or a temperature readout having a sensitivity of at least 0.1°C.Calibration should also be verified at 75mm above the upper face of the die.An alternate technique for calibration is to use a sheathed thermocouple or platinum-resistance temperature sensor with tip diameter of 9.460.1mm for insertion in the bore without material present.An example of this is shown in Fig.3.Calibration of the temperature-indicating device shall be verified at each run temperature.N OTE 9—The response of the temperature sensing device may be affected by immersion level.Take care to ensure adequate insulation of the device sensor and stabilization of the barrel temperature.5.5.3Heat shall be supplied by electric band heater(s)covering the entire length of the cylinder.The heater(s)may be single-or multi-element,depending upon the manufacturer’s control means.The heater(s)plus control system must be capable of maintaining the temperature within the required 60.2°C of the set point.The temperature sensor and readout equipment must be calibrated to a traceable national standard4Round-robin data showing flow rate and corresponding temperature profile of the melt obtained using probe-type thermocouples and platinum resistance tempera-ture sensors can be obtained from ASTM Headquarters.RequestRR:D20-1094.FIG.3Example of a Temperature Calibration DeviceNote A—Mineral glass insulation or equivalent spacer shall be bonded to tip and SS tube.Bond material shall be low conductivity type,400°C minimum rating.Insulation jacket material shall be low conductivity type (400°C minimum rating preferred,see Note 5).Note B—The RTD shall be inserted into bronze tip and bonded using high conductivity,400°C rated material.Tip of RTD element shall touch the bronze tip.Minimum insertion depth of 11.2mm clearance between RTD and tip wall shall beminimized.(that is,NIST)at least once per year.The cylinder with the heater(s)shall be lagged with38mm of foamed-glass insula-tion.An insulating plate3.2mm in thickness shall be attached to the bottom of the cylinder to minimize heat loss at this point.5.6Temperature Controller—The type of controller and sensor must be capable of meeting the required control tolerance specified in5.5.1.5.7Thermometer—Thermometers having a range of4°C graduated in0.2°C divisions may be used to indicate tempera-ture.The temperature at this point may not necessarily be the temperature of the material10mm above the die.The thermometer may be used to monitor indirectly the temperature of the material10mm above the die and may be calibrated by reference to a thermocouple or platinum resistance temperature sensor inserted in the material10mm above the die.See5.5.2 for a description of a method for measuring temperature. Warning—Caution should be observed with the use of a mercury-filled thermometer.Mercury vaporization occurs if the thermometer is broken.Mercury thermometers are not to be used at or above the boiling point of mercury,which is357°C. N OTE10—Warning:5.8Level—Provision shall be made for vertical alignment of the bore of the extrusion plastometer.This is necessary to minimize subtractive loads resulting from rubbing or friction between the piston tip and sidewall.Means of alignment are discussed in Appendix X1.5.9Accessory Equipment—Necessary accessories include equipment for charging samples to the cylinder,a funnel,a die plug,a tool for cutting off the extruded sample,a timer or stop watch,cleaning equipment,go/no-go gages,a balance accurate to60.001g,and,when required,a weight or weight-piston support.N OTE11—Satisfactory operation of the apparatus for polyethylenes can be ascertained by making measurements on NIST Standard Reference Materials(SRMs)certified for meltflow rate.The four SRMs certified under condition190/2.16are SRM1473with aflow rate of1.29g/min, SRM1474with aflow rate of5.03g/10min,SRM1496with aflow rate of0.26g/10min,and SRM1497with aflow rate of0.19g/10min.SRM 1475a is certified under condition190/3.25with aflow rate of2.20g/10 min.56.Test Specimen6.1The test specimen may be in any form that can be introduced into the bore of the cylinder,for example,powder, granules,strips offilm,or molded slugs.It may be desirable to preform or pelletize a powder.7.Conditioning7.1Many thermoplastic materials do not require condition-ing prior to testing.Materials which contain volatile compo-nents,are chemically reactive,or have other special character-istics most probably require appropriate conditioning procedures.Moisture not only affects reproducibility offlow rate measurement but,in some types of materials,degradation is accelerated by moisture at the high temperatures used in testing.Check the applicable material specification for any conditioning requirements before using this test.See Practice D618for appropriate conditioning practices.8.Procedural Conditions8.1Standard conditions of test are given in Table1.Test conditions shall be shown as:Condition___/___,where the temperature in degrees Celsius is shownfirst,followed by the weight in kilograms.For example:Condition190/2.16.8.2The following conditions have been found satisfactory for the material listed:Material Condition Acetals(copolymer and homopolymer)190/2.16190/1.05 Acrylics230/1.2230/3.8 Acrylonitrile-butadiene-styrene200/5.0230/3.8220/10Acrylonitrile/butadiene/styrene/polycarbonate230/3.8250/1.2blends265/3.8265/5.0 Cellulose esters190/0.325190/2.16190/21.60210/2.16 Ethylene-chlorotrifluoroethylene copolymer271.5/2.16Ethylene-tetrafluoroethylene copolymer297/5.0Nylon275/0.325235/1.0235/2.16235/5.0275/5.0Perfluoro(ethylene-propylene)copolymer372/2.16Perfluoroalkoxyalkane372/5.0Polycaprolactone125/2.1680/2.16 Polychlorotrifluorethylene265/12.5Polyether sulfone(PES)380/2.16360/10343/2.16Polyethylene125/0.325125/2.16250/1.2190/0.325190/2.16190/21.60190/10310/12.5Polycarbonate300/1.2Polymonochlorotrifluoroethylene265/21.6265/31.6Polypropylene230/2.16Polyphenyl sulfone(PPSU)365/5.0380/2.16 Polystyrene200/5.0230/1.2230/3.8190/5.0 Polysulfone(PSU)343/2.16360/10 Polyterephthalate250/2.16210/2.16285/2.16Poly(vinyl acetal)150/21.6Poly(vinylidenefluoride)230/21.6230/5.0Poly(phenylene sulfide)315/5.0Styrene acrylonitrile220/10230/10230/3.8Styrenic Thermoplastic Elastomer190/2.16200/5.0 Thermoplastic Elastomer-Ether-Ester190/2.16220/2.16230/2.16240/2.16250/2.16 Thermoplastic elastomers(TEO)230/2.16Vinylidenefluoride copolymers230/21.6230/5.0for T m=100°use120/5.0or21.6N OTE12—Some materials may require special materials of construc-tion or handling for performing this test.Please refer to the material specification for appropriate recommendations.8.3If more than one condition is used and the material is polyethylene,the determination of Flow Rate Ratio(FRR)has been found to be useful.The FRR is a dimensionless number derived by dividing theflow rate at Condition190/10by the flow rate at Condition190/2.16.N OTE13—When determining such a ratio offlow rates for a material at5These standard polyethylenes are available from the National Institute of Standards and Technology,Office of Standard Reference Materials,Washington,DC20234.the same temperature under different loads,it has been found that precision is maximized when one operator uses one Procedure (A or B),the same plastometer,and the same die for both measurements (the die need not be removed from the plastometer between the two determina-tions).9.Procedure A—Manual Operation9.1Select conditions of temperature and load from Table 1in accordance with material specifications such that flow rates will fall between 0.15to 50g/10min.9.2Ensure that the bore of the extrusion plastometer is properly aligned in the vertical direction.(See Appendix X1.)9.3Inspect the apparatus and die for cleanliness.If it is not clean,see 9.11.N OTE 14—The degree of cleanliness can significantly influence the flow rate results,therefore a thorough method of cleaning must be established.It has been found that three swabs of the barrel is satisfactory for most materials and that the die,barrel,and piston are more easily cleaned while hot.9.4Check the die bore diameter with appropriately sized no-go/go gages prior to testing.Make frequent checks to determine whether the die diameter (tested with die at 2365°C)is within the tolerances given in 5.3.N OTE 15—Cleaning and usage can result in a die diameter that is out of specifications.Data has shown that erroneous results will be obtained if the die diameter is not within the appropriate tolerances.9.5Verify that the temperature is stable and within 60.2°C of the appropriate test temperature as specified in 5.5.1.9.6Insert the die and the piston.The temperature of the cylinder with the piston and die in place must be stable at the appropriate test temperature 15min before testing is begun.When equipment is used repetitiously,it should not be neces-sary to heat the piston and die for 15min.N OTE 16—The reduction in heating time when the unit is being used repetitiously is only allowed when runs of the same or similar material are being measured over a continuous time frame.If the piston and die are removed and cleaned,they should be considered “cold”and the full 15minutes heating stabilization time required.9.7Remove the piston and place it on an insulated surface.Charge the cylinder within 1min with a weighed portion of the sample in accordance with the expected flow rate,as given in Table 2.Reinsert the piston and add the appropriate weight.N OTE 17—Experience has shown that for the best reproducibility the piston should operate within the same part of the cylinder for each measurement.The piston is scribed so the starting point for each extrusion is roughly the same.Some excess of material over the minimum required for the actual flow measurement portion of the test is provided by the charging weights shown in Table 2.This is necessary to achieve a void-free extrudate and flow equilibrium before start of rate measure-ments.N OTE 18—It is frequently helpful to take interim cuts of the extrudate at uniform time intervals during the specified extrusion time.Weights ofTABLE 1Standard Test Conditions,Temperature,and LoadCondition Temperature,°CTotal Load Including Piston,kgApproximate PressureStandard DesignationkPa psi 80/2.1680 2.16125/0.3251250.32544.8 6.5125/2.16125 2.16298.243.25150/2.16150 2.16298.243.25190/0.3251900.32544.8 6.5190/2.16190 2.16298.243.25190/21.6019021.602982.2432.5200/5.0200 5.0689.5100.0230/1.2230 1.2165.424.0230/3.8230 3.8524.076.0265/12.526512.51723.7250.0275/0.3252750.32544.8 6.5230/2.16230 2.16298.243.25190/1.05190 1.05144.721.0190/10.019010.01379.0200.0300/1.2300 1.2165.424.0190/5.0190 5.0689.5100.0235/1.0235 1.0138.220.05235/2.16235 2.16298.243.25235/5.0235 5.0689.5100.0250/2.16250 2.16298.243.25310/12.531012.51723.7250.0210/2.16210 2.16298.243.25285/2.16285 2.16298.243.25315/5.0315 5.0689.5100.0372/2.16372 2.16298.243.25372/5.0372 5.0689.5100297/5.0297 5.0689.5100230/21.623021.62982.2432.5230/5.0230 5.0689.5100265/21.626521.62982.2432.5265/31.626531.64361.2632.5271.5/2.16271.5 2.16298.243.25220/1022010.01379.0200.0250/1.2250 1.2165.424.0265/3.8265 3.8524.076.0265/52655.0689.5100.0these individual cuts give an indication of the presence of bubbles which may be masked due to their size or to opacity of the sample.This technique is particularly helpful in the case of highly pigmented materials.Forcing out some of the resin manually during the preheat period often eliminates bubbles in the test extrudate.9.8Allow time for the material to soften and begin to melt,and then purge some material to a position such that subse-quent travel of the piston will position the lower scribe mark at the reference start position 7.060.5min from the completion of the charge.Purge must be completed at least 2min prior to start of the test for materials having melt flow rates less than 10g/10min.N OTE 19—It has been found that purging within 60s of the start time will result in higher variability in the data.N OTE 20—There may be cases where the 7.060.5min is too much or not enough preheat time.For those materials,provisions must be in the material documents.It is necessary to refer to the appropriate material document before beginning any test.N OTE 21—Additional care may be necessary to prevent thermal degra-dation in the extrusion plastometer.This is sometimes done by the addition of an appropriate antioxidant.For highly unstable materials,it may be necessary to use alternative techniques as an indication of flow charac-teristics.9.9For materials with flow rates greater than 10g/10min,a weight (and if needed,a piston)support must be used after the initial purge.The support shall be removed at such a time as to allow the test to begin within 760.5min of the completion of the charge.The piston/weight support should be of such a length that the lower scribe mark of the supported piston/weight will be 25mm above the top of the guide bushing or other suitable reference mark.N OTE 22—It has been found that the effect of choosing plugging,weight support,or both,is significant to the flow rate results.The choice of piston support was made to cover all conditions and flow rates 10to 50g/10min.9.10For all tests,start collecting a timed extrudate when requirements for the piston position are met,provided this iswithin 7.060.5min from the end of charging;otherwise,discard the charge and repeat the test with readjusted piston position after the initial purge,or change weights.Require-ments are that the top scribed mark on the piston be visible above the cylinder or index and that the lower scribe mark be in the cylinder or below the index.As the lower scribed mark approaches the top of the cylinder or index,reset the timer to zero,then simultaneously start the timer and make the initial cut-off when the position requirements are met.Make the final cut-off exactly when the time interval given in Table 2is reached.Collect the timed extrudate.If the extrudate contains visible bubbles,discard the complete charge and begin the test again.N OTE 23—The charge weight should only be increased if no material is being purged and there is still not enough material to complete the test.9.11Discharge the remainder of the specimen and push the die out through the top of the cylinder.Swab out the cylinder with cloth patches after the manner of cleaning a pistol barrel.The die may be cleaned by dissolving the residue in a solvent.A better method is pyrolytic decomposition of the residue in a nitrogen atmosphere.Place the die in a tubular combustion furnace or other device for heating to 550610°C and clean with a small nitrogen purge through the die.This method is preferable to flame or solvent cleaning,being faster than solvent cleaning and less detrimental to the die than an open flame.In certain cases where materials of a given class having similar flow characteristics are being tested consecutively,interim die cleaning may be unnecessary.In such cases,however,the effect of cleaning upon flow rate determination must be shown to be negligible if this step is avoided.9.12Once the extrudate is cool,weigh to the nearest 1mg.9.13Multiply the weight of the extrudate by the appropriate factor shown in Table 2to obtain the flow rate in grams per 10min.N OTE 24—Frequently,errors in test technique,apparatus geometry,or test conditions,which defy all but the most careful scrutiny exist,causing discrepancy in flow rate determinations.The existence of such errors is readily determined by periodically measuring a reference sample of known flow rate.The flow rate value and range to be tolerated can be determined using a statistically correct test program composed of multiple determinations with various instruments.Standard samples of polyethyl-ene,linear or branched,are available from the National Institute of Standards and Technology.9.14In case a specimen has a flow rate at the borderline of the ranges in Table 2and slightly different values are obtained at different time intervals,the referee value shall be obtained at the longer time interval.10.Procedure B—Automatically Timed Flow RateMeasurement 10.1Apparatus :10.1.1Extrusion plastometer and auxiliary equipment are detailed in Section 4and below.10.1.2A timing device shall electrically,optically,or me-chanically time piston movement within the specified travel range.The requirements of the system are as follows:10.1.2.1Sense and indicate the piston travel time within 60.01s (see Note 1).TABLE 2Standard Test Conditions,Sample Mass,A and TestingTime BFlow Range,g/10min Suggested Mass of Sample in Cylinder,gTime Inter-val,min Factor for Obtaining Flow Rate in g/10min0.15to 1.0 2.5to 3.0 6.00 1.67>1.0to 3.5 3.0to 5.0 3.00 3.33>3.5to 10 4.0to 8.0 1.0010.00>10to 25 4.0to 8.00.5020.00>254.0to 8.00.2540.00AThis is a suggested mass for materials with melt densities of about 0.7g/cm 3.Correspondingly,greater quantities are suggested for materials of greater melt densities.Density of the molten resin (without filler)may be obtained using the procedure described by Terry,B.W.,and Yang,K.,“A New Method for Determining Melt Density as a Function of Pressure and Temperature,”SPE Journal ,SPEJA,Vol.20,No.6,June 1964,p.540or the procedure described by Zoller,Paul,“The Pressure-Volume-Temperature Properties of Polyolefins,”Journal of Applied Poly-mer Science ,Vol 23,1979,p.1051.It may also be obtained from the weight of an extruded known volume of resin at the desired temperature.For example,25.4mm (1in.)of piston movement extrudes 1.804cm 3of resin.An estimate of the density of the material can be calculated from the following equation:resin density at test temperature 5M /1.804where:M =mass of extruded resin.BSee9.14.。

附录A外文翻译Why Plastic Flows Better in Aluminum InjectionMoldsAn investigative study directly comparing melt flow characteristics of general purpose resins in QC-10 aluminum molds and P20 steel molds.Part 1 IntroductionThere have been numerous articles published regarding the cycle time advantage aluminum molds have over steel when configured with the same gate, part geometry and cooling channels, but there is little specific information available to demonstrate why this happens and how it improves the injection mold process.Alcoa Forge and Cast Products teamed up with Aluminum Injection Mold Co. (Rochester, NY) and sponsored a case study to uncover the differences known to exist when molding thermoplastics in aluminum versus steel molds. The key objectives were to quantify the differences by comparing how thermoplastics react in an aluminum mold versus a steel one, measure those differences, and share the results of the experiment. The results should help mold makers and molders better understand the potential savings and improvements for molding plastic components in aluminum tools, specifically addressing how:1) Plastic material flows longer distances with less injection pressure, when compared to steel2) Molds fill faster and more efficiently3) Parts have minimal warp and better dimensional stability Aluminum’s thermal conductivity is nearly 5 times greater than that of steel (table 1). In a 2002 article published in Moldmaking Technology, Douglas Bryce discusses an IBM tooling study comparing identical aluminum and steel molds producing the same plastic components over a five year period. The article suggested that the aluminum molds cost up to 50% less to build and can be delivered in one half the time. It went on to say these tools produced higher quality products having cycle times that were 25 to 40% less than the steel molds.In 2005, an article written in the Moldflow publication, Flowfront, looked at computer simulation of cycle time and cooling versus actual molding. After carrying out simulations on 12 parts which had very different characteristics in terms of shape, size and plastic materials, it was concluded that significant savings in total cycle time could be realized by using aluminum instead of steel molds. Cycle time savings of 10-20% were seen in cases where there were no critical tolerances linked to the deformation of the part due to the effect of the heat. However, savings of 60-200% were seen in cases where heat deformation affected critical design tolerance levels.Studies like these are relevant to the industry and this case study looks at the basis of why plastic flows better in aluminum.Part 2 ToolingSpiral test molds, built in accordance to ASTM D3123-98 were selected for the tool design. This shape would standardize the channel length, size of overall mold, cooling and gate location. In addition, each mold was fitted with a series of 4 thermocouples to monitor and document, in real time, what the metal does when injected with molten plastic. All the thermocouples were connected to a data logger and computer for data collection. For the aluminum molds, we used QC-10 mold plate and for the steel molds, P20. Six molds ofidentical geometry were built - three in QC-10 and three in P20. The spiral mold shape was sized at 6 mm wide and channel depths of 1 mm, 2mm and 3mm, respectively. The sizes of the tools were a standard 7" x 8" master unit die and all the mold plates were the same thicknesses. (Fig. 1)The sprue diameter was identically sized for each of the six unit molds. Identical water lines were drilled to complete the cooling circuits. Four of the six molds, the 1 mm and 2 mm molds in both materials, were fitted with thermocouples that came in from the back and were approximately 0.5 mm from the cavity surface. On the 3 mm spiral unit molds, a 5th thermocouple was placed into secondary vent area to monitor the vent temperature during molding. All six molds were laser engraved on the "A" side in inch increments from 1" to 67". The surfaces were finished with a 600 grit stone. The test was set up in a 55 ton Toyo injection mold machine.Seven unfilled, general purpose thermoplastic resins were selected for this trial: polyethylene, polypropylene, polystyrene, PE, PC/PE, nylon, and polycarbonate.Part 3 FindingsThe QC-10 molds heated five times faster than the P20 molds, as we set up to run each trial. Across all the trials, the QC-10 mold temperature stayed consistently within 1-3 degrees of the mold temperature set point. During the inject phase, a temperature spike of 10-20 degrees with an abrupt return to set point was observed.The P20 mold temperature stayed consistently 10-25 degrees above mold temperature set point. During the inject phase, additional increases of 15-30 degrees were observed before slowly trending downward. When using the QC-10 molds, we did not see an appreciable change in cycle time, part to part, even when we ran the materials at the high end of the manufacturers recommended melt/mold temperatures. However, the P20 molds continued to get hotter and the cycle time became even longer.In view of these findings, it is not surprising that there are some plastic consultants extolling the virtues of running plastic resin as much as 100 degrees below the manufacturer's recommended settings when using P20 or other steel injection molds, even though doing so could void the manufacturer's guarantees.Part 4 ConclusionThe results of this experiment were both a surprise and not a surprise.We were not surprised to prove what we set out to prove, but the road that led us there was an unexpected one. We were pleased to show that plastic partsmolded in aluminum would minimize warp and enhance dimensional stability, allow molds to fill faster and more efficiently and allow plastic material to flow greater distances with less injection pressure when compared to steel. We demonstrated that using aluminum gives the benefit of making molds less expensive to produce, shortening mold delivery time, producing higher quality molded plastic parts and enabling the realization of producing more plastic parts per day.The surprise in the experiment was that the expected results were achieved in a different, unexpected way. We thought we would arrive at the desired results because aluminum molds would take on heat from the hot melt during the injection phase, enabling the plastic to fill the mold cavity more quickly with less pressure and less density change. Conversely, we felt that the steel molds would take on less heat, thereby creating more "skinning", and restricting the flow front resulting in the need for higher injection pressure and causing density changes from the gate to the longest flow length.What we actually found was that the QC-10 did not take on or hold as much heat as we previously thought, thus allowing the molten plastic to move in quickly and quench quickly, therefore there was not a density change due to excess injection pressure. We discovered that the steel actually took on and held much more heat. During the inject phase, plastic filled the cavity and stayed molten much longer allowing for additional inject pressure which caused density changes before solidification. We hope the information provided in this paper adds to the knowledge base used to consider aluminum as a choice for your next production injection mold.为什么在铝制注塑模中塑料的流动性更好一项调查研究直接比较了树脂在10铝模具和P20钢模具中时的融化流动特性。

分子量分布系数英文Molecular Weight Distribution CoefficientOne of the fundamental characteristics of polymeric materials is their molecular weight distribution (MWD), which is a critical parameter that significantly impacts the physical and mechanical properties of the final product. The molecular weight distribution coefficient, also known as the polydispersity index (PDI), is a measure of the breadth or heterogeneity of the molecular weight distribution.The molecular weight distribution of a polymer is typically represented by a Gaussian or normal distribution curve, where the x-axis represents the molecular weight and the y-axis represents the relative abundance or frequency of the various molecular weight species. The shape of this curve can provide valuable insights into the synthesis and processing of the polymer.The polydispersity index is a dimensionless quantity that is calculated by dividing the weight-average molecular weight (Mw) by the number-average molecular weight (Mn) of the polymer. This ratio provides a measure of the distribution of molecular weights within the polymer sample. A PDI value of 1 indicates a perfectlymonodisperse system, where all polymer chains have the same molecular weight. In contrast, a higher PDI value (greater than 1) suggests a broader distribution of molecular weights, with a greater range of chain lengths present in the sample.The polydispersity index can have a significant impact on the properties of a polymer. Polymers with a narrow molecular weight distribution (low PDI) tend to exhibit better mechanical properties, such as higher tensile strength and impact resistance, as well as improved processability. This is because the polymer chains are more uniform in length, allowing for more efficient packing and better stress transfer within the material.On the other hand, polymers with a broader molecular weight distribution (high PDI) can exhibit enhanced melt flow properties, which can be advantageous in certain processing techniques, such as injection molding or extrusion. The presence of shorter chains can improve the flow characteristics of the polymer melt, allowing for better filling of molds or die cavities.The polydispersity index is a crucial parameter in the characterization and optimization of polymer synthesis processes. It can be influenced by various factors, such as the polymerization mechanism, the presence of chain transfer agents, and the reaction conditions. For example, step-growth polymerizations, such as polycondensation,typically result in broader molecular weight distributions and higher PDI values compared to chain-growth polymerizations, such as free radical or anionic polymerization.In addition to the impact on physical and mechanical properties, the molecular weight distribution can also affect the rheological behavior of polymers, which is particularly important in processing operations. Polymers with a broader MWD tend to exhibit shear-thinning behavior, where the viscosity decreases with increasing shear rate. This can be advantageous in certain processing techniques, such as extrusion or injection molding, where the polymer needs to flow easily through the die or mold.To determine the polydispersity index of a polymer, various analytical techniques can be employed, such as size-exclusion chromatography (SEC) or gel permeation chromatography (GPC). These techniques separate the polymer chains based on their size or hydrodynamic volume, allowing for the determination of the number-average and weight-average molecular weights, and subsequently, the calculation of the PDI.In conclusion, the molecular weight distribution coefficient, or polydispersity index, is a critical parameter in the characterization and understanding of polymeric materials. It provides valuable insights into the synthesis, processing, and ultimate performance ofpolymers, making it an essential tool in the development and optimization of various polymer-based products.。

高分子物理名词解释Θ溶剂(Θ solvent):链段-溶剂相互吸引刚好抵消链段间空间排斥的溶剂,形成高分子溶液时观察不到远程作用,该溶剂中的高分子链的行为同无扰链2.7Θ温度(Θ temperature):溶剂表现出Θ溶剂性质的温度2.7Argon理论(Argon theory):一种银纹扩展过程的模型,描述了分子链被伸展将聚合物材料空化的过程5.3Avrami方程(Avrami equation):描述物质结晶转化率与时间关系的方程:--α,α为转化率,K与n称Avrami常数(Avrami constants) 4.8 =Kt1n)ex p(Bingham流体(Bingham liquid):此类流体具有一个屈服应力σy,应力低于σy时不产生形变,当应力大于σy时才发生流动,应力高于σy的部分与应变速率呈线性关系3.13 Boltzmann叠加原理(Blotzmann superposition principle):Boltzmann提出的粘弹性原理:认为样品在不同时刻对应力或应变的响应各自独立并可线性叠加 3.8Bravais晶格(Bravais lattice):结构单元在空间的排列方式4.1Burger's模型(Burger's model):由一个Maxwell模型和一个Kelvin模型串联构成的粘弹性模型3.7Cauchy应变(Cauchy strain):拉伸引起的相对于样品初始长度的形变分数,又称工程应变3.16Charpy冲击测试(Charpy impact test):样品以简支梁形式放置的冲击强度测试,测量样品单位截面积的冲击能5.4Considère构图(Considère construction):以真应力对工程应作图以判定细颈稳定性的方法5.2Eyring模型(Eyring model):一种描述材料形变过程的分子模型,认为形变是结构单元越过能垒的跳跃式运动5.2Flory-Huggins参数(Flory-Huggins interaction parameter):描述聚合物链段与溶剂分子间相互作用的参数,常用χ表示,物理意义为一个溶质分子被放入溶剂中作用能变化与动能之比2.11.2Flory构图(Flory construction):保持固定拉伸比所需的力f对实验温度作图得到,由截距确定内能对拉伸力的贡献,由斜率确定熵对拉伸力的贡献2.16.2Flory特征比(characteristic ratio):无扰链均方末端距与自由连接链均方末端距的比值2.4 Griffith理论(Griffith theory):一种描述材料断裂机理的理论,认为断裂是吸收外界能量产生新表面的过程5.4Hencky应变(Hencky strain):拉伸引起的相对于样品形变分数积分,又称真应变3.16 Hermans取向因子(Hermans orientation factor):描述结构单元取向程度的参数,是结构单元与参考方向夹角余弦均方值的函数4.8, 4.10Hoffman-Weeks作图法(Hoffman-Weeks plot):一种确定平衡熔点的方法。
英汉对照ISO 21809-4:2009石油管道2PE外防腐层国际技术标准编译王向农(中国石油工程建设协会防腐保温技术专业委员会)2012年5月前言2009年11月15日,国际标准化组织(ISO)颁布了ISO 21809-4:2009石油天然气管道两层聚乙烯外防腐层技术标准第1版。
标准文件ISO 21809-4:2009规定了符合标准文件ISO 13623定义的石油天然气工业管道输送系统用的裸钢管,在防腐厂涂敷两层聚乙烯管道外防腐层的材料的质量评定、涂敷、检验、测试、装卸和储存的技术要求。
本标准文件是由ISO/TC 67石油天然气工业技术委员会下属的SC 2管道输送系统分会按照ISO/IEC指令第2部分规定编制的。
本文仅为笔者学习标准文件ISO21809-4:2009的学习心得,本文采用英汉对照形式,以便与业内同行探讨研究,译文完全属于笔者自己的解读。
标准文件ISO21809-4:2009的附录包括七项与本标准内容有关的检验和试验方法,附录是本标准文件不可分割的一部分。
执行ISO 21809-4:2009标准必须严格遵照英文原版的ISO国际标准,详情可访问国际标准化组织官方网站。
ISO 21809-4:2009Petroleum and natural gas industries - External coatings for buried or submerged pipelines used in pipeline transportation systems- Part 4: Polyethylene coatings(2-layer PE)石油天然气工业–管道输送系统中采用的埋地管道或水下管道的外防腐层–第四部分:聚乙烯外防腐层(两层聚乙烯)2009年11月15日第一版Contents目录1.0 Scope 范围2.0 Normative reference参照标准3.0 Terms and definitions 术语和定义4.0 Abbreviations terms缩写词汇5.0 General requirements 一般性要求5.1 Rounding 四舍五入5.2 Compliance with this part of ISO 21809执行本部分标准的保障6.0 Information supplied by the purchaser 采购方应当提供的资料6.1 General information 一般性资料6.2Additional information 额外的资料7.0Coating classification 防腐层的类别等级划分7.1 General 总则7.2 Coating systems 防腐层系统7.3Coating thickness classes 防腐层的厚度等级8.0 Materials 材料8.1 Pipe 管材8.2 Coating materials 防腐涂料8.3 Packaging 包装9.0 Coating qualification 防腐涂料的质量评定10.0 Application 涂敷10.1 General 总则10.2 Surface preparation 表面预处理10.3 Pipe temperature 管子温度10.4 Application of adhesive 涂敷胶粘剂10.5 Application of polyethylene 涂敷聚乙烯10.6 Cutback 管端焊接预留段11.0 Inspection and testing 检验和试验11.1 Inspection 检验11.2 Testing 试验12.0 Repair of coated pipe 防腐管段的修补12.1 Rectification of below-thickness coating 偏薄防腐层的纠正12.2 Repair 修补12.3 Stripping and recoating 剥去不合格的防腐层和重新涂敷13.0 Marking 标记13.1 General 总则13.2 Required markings 需要的标记14.0 Handling and storage in the coating area 涂敷作业区里搬运与储存14.1 Handling 搬运14.2 Storage 储存15.0 Test report and certificate of compliance 试验报告和合格证书1.0 Scope 范围This part of ISO 21809 specifies the requirements for qualification, application, inspection, testing, handling and storage of materials for plant application of two-layer polyethylene coatings (2-layer PE) applied externally for the corrosion protection of bare steel pipe for use in pipeline transportation systems for the petroleum and natural gas industries as defined in ISO 13623.标准文件ISO 21809的这一部分内容规定了符合标准文件ISO 13623定义的石油天然气工业管道输送系统用的裸钢管,在防腐厂涂敷两层聚乙烯管道外防腐层的材料的质量评定、涂敷、检验、测试、装卸和储存的技术要求。
半导体一些术语的中英文对照离子注入机ionimplanterLSS理论LindhandScharffandSchiotttheory 又称“林汉德-斯卡夫-斯高特理论”。
沟道效应channelingeffect射程分布rangedistribution深度分布depthdistribution投影射程projectedrange负性光刻胶negativephotoresist正性光刻胶positivephotoresist无机光刻胶inorganicresist多层光刻胶multilevelresist电子束光刻胶electronbeamresistX射线光刻胶X-rayresist刷洗scrubbing甩胶spinning涂胶photoresistcoating后烘postbaking光刻photolithographyX射线光刻X-raylithography电子束光刻electronbeamlithography离子束光刻ionbeamlithography深紫外光刻deep-UVlithography光刻机maskaligner投影光刻机projectionmaskaligner曝光exposure接触式曝光法contactexposuremethod接近式曝光法proximityexposuremethod光学投影曝光法opticalprojectionexposuremethod磷硅玻璃phosphorosilicateglass硼磷硅玻璃boron-phosphorosilicateglass钝化工艺passivationtechnology 多层介质钝化multilayerdielectricpassivation划片scribing电子束切片electronbeamslicing烧结sintering印压indentation热压焊thermocompressionbonding热超声焊thermosonicbonding冷焊coldwelding点焊spotwelding球焊ballbonding楔焊wedgebonding内引线焊接innerleadbonding外引线焊接outerleadbonding梁式引线beamlead装架工艺mountingtechnology附着adhesion封装packaging金属封装metallicpackagingAmbipolar双极的Ambienttemperature环境温度Amorphous无定形的,非晶体的Amplifier功放扩音器放大器Analogue(Analog)comparator模拟比较器Angstrom埃Anneal退火Anisotropic各向异性的Anode阳极Arsenic(AS)砷Auger俄歇Augerprocess俄歇过程Avalanche雪崩Avalanchebreakdown雪崩击穿Avalancheexcitation雪崩激发Backgroundcarrier本底载流子Backgrounddoping本底掺杂Backward反向Backwardbias反向偏置Ballastingresistor整流电阻Ballbond球形键合Band能带Bandgap能带间隙Barrier势垒Barrierlayer势垒层Barrierwidth势垒宽度Base基极Basecontact基区接触Basestretching基区扩展效应Basetransittime基区渡越时间Basetransportefficiency基区输运系数Base-widthmodulation基区宽度调制Basisvector基矢Bias偏置Bilateralswitch双向开关Binarycode二进制代码Binarycompoundsemiconductor二元化合物半导体Bipolar双极性的BipolarJunctionTransistor(BJT)双极晶体管Bloch布洛赫Blockingband阻挡能带Chargeconservation电荷守恒Chargeneutralitycondition电中性条件Chargedrive/exchange/sharing/transfer/storage电荷驱动/交换/共享/转移/存储Chemmicaletching化学腐蚀法Chemically-Polish化学抛光Chemmically-MechanicallyPolish(CMP)化学机械抛光Chip芯片Chipyield芯片成品率Clamped箝位Clampingdiode箝位二极管Cleavageplane解理面Clockrate时钟频率Clockgenerator时钟发生器Clockflip-flop时钟触发器Close-packedstructure密堆积结构Close-loopgain闭环增益Collector集电极Collision碰撞CompensatedOP-AMP补偿运放Common-base/collector/emitterconnection共基极/集电极/发射极连接Common-gate/drain/sourceconnection共栅/漏/源连接Common-modegain共模增益Common-modeinput共模输入Common-moderejectionratio(CMRR)共模抑制比Compatibility兼容性Compensation补偿Compensatedimpurities补偿杂质Compensatedsemiconductor补偿半导体ComplementaryDarlingtoncircuit互补达林顿电路ComplementaryMetal-Oxide-SemiconductorField-Effect-Transistor(CMOS)互补金属氧化物半导体场效应晶体管Complementaryerrorfunction余误差函数Computer-aideddesign(CAD)/test(CAT)/manufacture(CAM)计算机辅助设计/测试/制De.broglie德布洛意Decderate减速Decibel(dB)分贝Decode译码Deepacceptorlevel深受主能级Deepdonorlevel深施主能级Deepimpuritylevel深度杂质能级Deeptrap深陷阱Defeat缺陷Degeneratesemiconductor简并半导体Degeneracy简并度Degradation退化DegreeCelsius(centigrade)/Kelvin摄氏/开氏温度Delay延迟Density密度Densityofstates态密度Depletion耗尽Depletionapproximation耗尽近似Depletioncontact耗尽接触Depletiondepth耗尽深度Depletioneffect耗尽效应Depletionlayer耗尽层DepletionMOS耗尽MOSDepletionregion耗尽区Depositedfilm淀积薄膜Depositionprocess淀积工艺Designrules设计规则Die芯片(复数dice)Diode二极管Dielectric介电的Dielectricisolation介质隔离Difference-modeinput差模输入Differentialamplifier差分放大器Differentialcapacitance微分电容Diffusedjunction扩散结Diffusion扩散Diffusioncoefficient扩散系数Diffusionconstant扩散常数Diffusivity扩散率Diffusioncapacitance/barrier/current/furnace扩散电容/势垒/电流/炉Electrostatic静电的Element元素/元件/配件Elementalsemiconductor元素半导体Ellipse椭圆Ellipsoid椭球Emitter发射极Emitter-coupledlogic发射极耦合逻辑Emitter-coupledpair发射极耦合对Emitterfollower射随器Emptyband空带Emittercrowdingeffect发射极集边(拥挤)效应Endurancetest=lifetest寿命测试Energystate能态Energymomentumdiagram能量-动量(E-K)图Enhancementmode增强型模式EnhancementMOS增强性MOSEntefic(低)共溶的Environmentaltest环境测试Epitaxial外延的Epitaxiallayer外延层Epitaxialslice外延片Expitaxy外延Equivalentcurcuit等效电路Equilibriummajority/minoritycarriers平衡多数/少数载流子ErasableProgrammableROM(EPROM)可搽取(编程)存储器Errorfunctioncomplement余误差函数Etch刻蚀Etchant刻蚀剂Etchingmask抗蚀剂掩模Excesscarrier过剩载流子Excitationenergy激发能Excitedstate激发态Exciton激子Extrapolation外推法Extrinsic非本征的Extrinsicsemiconductor杂质半导体Face-centered面心立方Falltime下降时间Heatsink散热器、热沉Heavy/lightholeband重/轻空穴带Heavysaturation重掺杂Hell-effect霍尔效应Heterojunction异质结Heterojunctionstructure异质结结构HeterojunctionBipolarTransistor(HBT)异质结双极型晶体Highfieldproperty高场特性High-performanceMOS.(H-MOS)高性能MOS.Hormalized归一化Horizontalepitaxialreactor卧式外延反应器Hotcarrior热载流子Hybridintegration混合集成Image-force镜象力Impactionization碰撞电离Impedance阻抗Imperfectstructure不完整结构Implantationdose注入剂量Implantedion注入离子Impurity杂质Impurityscattering杂志散射Incrementalresistance电阻增量(微分电阻)In-contactmask接触式掩模Indiumtinoxide(ITO)铟锡氧化物Inducedchannel感应沟道Infrared红外的Injection注入Inputoffsetvoltage输入失调电压Insulator绝缘体InsulatedGateFET(IGFET)绝缘栅FETIntegratedinjectionlogic集成注入逻辑Integration集成、积分Interconnection互连Interconnectiontimedelay互连延时Interdigitatedstructure交互式结构Interface界面Interference干涉Internationalsystemofunions国际单位制Internallyscattering谷间散射Matching匹配Maxwell麦克斯韦Meanfreepath平均自由程Meanderedemitterjunction梳状发射极结Meantimebeforefailure(MTBF)平均工作时间Megeto-resistance磁阻Mesa台面MESFET-MetalSemiconductor金属半导体FETMetallization金属化Microelectronictechnique微电子技术Microelectronics微电子学Millenindices密勒指数Minoritycarrier少数载流子Misfit失配Mismatching失配Mobileions可动离子Mobility迁移率Module模块Modulate调制Molecularcrystal分子晶体MonolithicIC单片ICMOSFET金属氧化物半导体场效应晶体管Mos.Transistor(MOST)MOS.晶体管Multiplication倍增Modulator调制Multi-chipIC多芯片ICMulti-chipmodule(MCM)多芯片模块Multiplicationcoefficient倍增因子Nakedchip未封装的芯片(裸片)Negativefeedback负反馈Negativeresistance负阻Nesting套刻Negative-temperature-coefficient负温度系数Noisemargin噪声容限Nonequilibrium非平衡Nonrolatile非挥发(易失)性Normallyoff/on常闭/开Numericalanalysis数值分析Occupiedband满带Officienay功率Photoelectriccell光电池Photoelectriceffect光电效应Photoenicdevices光子器件Photolithographicprocess光刻工艺(photo)resist(光敏)抗腐蚀剂Pin管脚Pinchoff夹断PinningofFermilevel费米能级的钉扎(效应)Planarprocess平面工艺Planartransistor平面晶体管Plasma等离子体Plezoelectriceffect压电效应Poissonequation泊松方程Pointcontact点接触Polarity极性Polycrystal多晶Polymersemiconductor聚合物半导体Poly-silicon多晶硅Potential(电)势Potentialbarrier势垒Potentialwell势阱Powerdissipation功耗Powertransistor功率晶体管Preamplifier前置放大器Primaryflat主平面Principalaxes主轴Print-circuitboard(PCB)印制电路板Probability几率Probe探针Process工艺Propagationdelay传输延时Pseudopotentialmethod膺势发Punchthrough穿通Pulsetriggering/modulating脉冲触发/调制Pulse WidenModulator(PWM)脉冲宽度调制Punchthrough穿通Push-pullstage推挽级Qualityfactor品质因子Quantization量子化Schottkybarrier肖特基势垒Schottkycontact肖特基接触Schrodingen薛定厄Scribinggrid划片格Secondaryflat次平面Seedcrystal籽晶Segregation分凝Selectivity选择性Selfaligned自对准的Selfdiffusion自扩散Semiconductor半导体Semiconductor-controlledrectifier可控硅Sendsitivity灵敏度Serial串行/串联Seriesinductance串联电感Settletime建立时间Sheetresistance薄层电阻Shield屏蔽Shortcircuit短路Shotnoise散粒噪声Shunt分流Sidewallcapacitance边墙电容Signal信号Silicaglass石英玻璃Silicon硅Siliconcarbide碳化硅Silicondioxide(SiO2)二氧化硅SiliconNitride(Si3N4)氮化硅SiliconOnInsulator绝缘硅Siliverwhiskers银须Simplecubic简立方Singlecrystal单晶Sink沉Skineffect趋肤效应Snaptime急变时间Sneakpath潜行通路Sulethreshold亚阈的Solarbattery/cell太阳能电池Solidcircuit固体电路SolidSolubility固溶度Sonband子带Transistoraging(stress)晶体管老化Transittime渡越时间Transition跃迁Transition-metalsilica过度金属硅化物Transitionprobability跃迁几率Transitionregion过渡区Transport输运Transverse横向的Trap陷阱Trapping俘获Trappedcharge陷阱电荷Trianglegenerator三角波发生器Triboelectricity摩擦电Trigger触发Trim调配调整Triplediffusion三重扩散Truthtable真值表Tolerahce容差Tunnel(ing)隧道(穿)Tunnelcurrent隧道电流Turnover转折Turn-offtime关断时间Ultraviolet紫外的Unijunction单结的Unipolar单极的Unitcell原(元)胞Unity-gainfrequency单位增益频率Unilateral-switch单向开关Vacancy空位Vacuum真空Valence(value)band价带Valuebandedge价带顶Valencebond价键Vapourphase汽相Varactor变容管Varistor变阻器Vibration振动Voltage电压Wafer晶片Waveequation波动方程Waveguide波导Wavenumber波数CT:ContaminationThreshold??污染阀值Ctrl:Control控制;管理;抑制D:Die芯片DAC igitalAnalogConverter??数字转换器DSP igitalSignalProcessing数字信号处理EFO:ElevtronicFlame-Off电子打火系统FA:FaceAngle顶锥角(面锥角)FAB:FreeAirBall空气球FD:FloppyDisk软盘,软式磁碟片Frd:Forward??前进GEM:GenericHi:HightMagnification高倍率Hybd:Hybrid混合动力/混合式Impd:Impedence阻抗Ins:Inspection检查,检验L/F eadFrame框架Lo:LowMagnification低倍率PM reventiveMaintenance??PR atternRecognitionT/P:TopPlate??顶板UPH:UnitPerHour??每小时产量UTI:UltrasonicTransducerInterface超声波传感受器接口VLL:VisualLeadLocator导脚定位W/C:WireClamp??线夹W/H:WorkHolder??轨道W/S:WireSpool??线轴ESD:ElectroStaticDischarge静电释放EPa:ESDProtectedarea??静电防护区ESDS??????????????????????静电敏感设备BM:BreakdownMaintenance事后维修CM:CorrectiveMaintenance改良保养PVM:PreventiveMaintenance预防保养MP:MaintencePreventive保养预防PM:ProductionMaintenance生产保养BG:backgrinding??背部研磨DS:diesaw????将wafer切die DA:dieattach??=DB:diebond??装片WB:wirebond焊线????。
第 29 卷第 1 期分析测试技术与仪器Volume 29 Number 1 2023年3月ANALYSIS AND TESTING TECHNOLOGY AND INSTRUMENTS Mar. 2023大型仪器功能开发(30 ~ 36)正负压一体式无空气X射线光电子能谱原位转移仓的开发及研制章小余,赵志娟,袁 震,刘 芬(中国科学院化学研究所,北京 100190)摘要:针对空气敏感材料的表面分析,为了获得更加真实的表面组成与结构信息,需要提供一个可以保护样品从制备完成到分析表征过程中不接触大气环境的装置. 通过使用O圈密封和单向密封柱,提出一种简便且有效的设计概念,自主研制了正负压一体式无空气X射线光电子能谱(XPS)原位转移仓,用于空气敏感材料的XPS测试,利用单向密封柱实现不同工作需求下正负压两种模式的任意切换. 通过对空气敏感的金属Li片和CuCl粉末进行XPS分析表明,采用XPS原位转移仓正压和负压模式均可有效避免样品表面接触空气,保证测试结果准确可靠,而且采用正压密封方式转移样品可以提供更长的密封时效性. 研制的原位转移仓具有设计小巧、操作简便、成本低、密封效果好的特点,适合给有需求的用户开放使用.关键词:空气敏感;X射线光电子能谱;原位转移;正负压一体式中图分类号:O657; O641; TH842 文献标志码:B 文章编号:1006-3757(2023)01-0030-07 DOI:10.16495/j.1006-3757.2023.01.005Development and Research of Inert-Gas/Vacuum Sealing Air-Free In-Situ Transfer Module of X-Ray Photoelectron SpectroscopyZHANG Xiaoyu, ZHAO Zhijuan, YUAN Zhen, LIU Fen(Institute of Chemistry Chinese Academy of Sciences, Beijing 100190, China)Abstract:For the surface analysis of air sensitive materials, and from the sample preparation to characterization, it is necessary to provide a device that can protect samples from exposing to the atmosphere environment so as to obtain accurate and impactful data of the surface chemistry. Through the use of O-ring and one-way sealing, a simple and effective design concept has been demonstrated, and an inert-gas/vacuum sealing air-free X-ray photoelectron spectroscopic (XPS) in-situ transfer module has been developed to realize the XPS analysis of air sensitive materials. The design of one-way sealing was achieved conveniently by switching between inert-gas and vacuum sealing modes in face of different working requirements. The XPS analysis of air-sensitive metal Li sheets and CuCl powders showed that both the sealing modes (an inert-gas/vacuum sealing) of the XPS in-situ transfer module can effectively avoid air contact on the sample surface, and consequently, can ensure the accuracy and reliability of XPS data. Furthmore, the inert gas sealing mode can keep the sample air-free for a longer time. The homemade XPS in-situ transfer module in this work is characterized by a compact design, convenient operation, low cost and effective sealing, which is suitable for the open access to the users who need it.收稿日期:2022−12−07; 修订日期:2023−01−17.基金项目:中国科学院化学研究所仪器孵化项目[Instrument and Device Functional Developing Project of Institute of Chemistry Chinese Academy of Sciences]作者简介:章小余(1986−),女,硕士,工程师,主要研究方向为电子能谱技术及材料表面分析,E-mail:xyiuzhang@ .Key words:air-sensitive;X-ray photoelectron spectroscopy;in-situ transfer;inert-gas/vacuum sealingX射线光电子能谱(XPS)是一种表面灵敏的分析技术,通常用于固体材料表面元素组成和化学态分析[1]. 作为表面分析领域中最有效的方法之一,XPS广泛应用于纳米科学、微电子学、吸附与催化、环境科学、半导体、冶金和材料科学、能源电池及生物医学等诸多领域[2-3]. 其中在催化和能源电池材料分析中,有一些样品比较特殊,比如碱金属电池[4-6]、负载型纳米金属催化剂[7-8]和钙钛矿材料[9]对空气非常敏感,其表面形态和化学组成接触空气后会迅速发生改变,直接影响采集数据的准确性和有效性,因此这类样品的表面分析测试具有一定难度. 目前,常规的光电子能谱仪制样转移过程通常是在大气环境中,将样品固定在标准样品台上,随后放入仪器进样室内抽真空至1×10−6 Pa,再转入分析室内进行测试. 这种制备和进样方式无法避免样品接触大气环境,对于空气敏感材料,其表面很容易与水、氧发生化学反应,导致无法获得材料表面真实的结构信息.为了保证样品表面状态在转移至能谱仪内的过程中不受大气环境影响,研究人员采用了各种技术来保持样品转移过程中隔绝空气. 比如前处理及反应装置与电子能谱仪腔室间真空传输[10-12]、外接手套箱 [13-14]、商用转移仓[15-16]、真空蒸镀惰性金属比如Al层(1.5~6 nm)[17]等. 尽管上述技术手段有效,但也存在一些缺点,例如配套装置体积巨大、试验过程不易操作、投入成本高等,这都不利于在普通实验室内广泛应用. 而一些电子能谱仪器制造商根据自身仪器的特点也研发出了相应配套的商用真空传递仓,例如Thermofisher公司研发的一种XPS 真空转移仓,转移过程中样品处于微正压密封状态,但其价格昂贵,体积较大,转移过程必须通过手套箱大过渡舱辅助,导致传递效率低,单次需消耗至少10 L高纯氩气,因此购置使用者较少,利用率低.另外有一些国内公司也研发了类似的商品化气体保护原位传递仓,采用微正压方式密封转移样品,但需要在能谱仪器进样室舱门的法兰上外接磁耦合机械旋转推拉杆,其操作复杂且放置样品的有效区域小,单次仅可放置尺寸为3 mm×3 mm的样品3~4个,进样和测试效率较低. 因此,从2016年起本实验团队开始自主研制XPS原位样品转移装置[18],经过结构与性能的迭代优化[19],最终研制出一种正负压一体式无空气XPS原位转移仓[20](本文简称XPS原位转移仓),具有结构小巧、操作便捷、成本低、密封效果好、正压和负压密封两种模式转移样品的特点. 为验证装置的密封时效性能,本工作选取两种典型的空气敏感材料进行测试,一种是金属Li材料,其化学性质非常活泼,遇空气后表面迅速与空气中的O2、N2、S等反应导致表面化学状态改变. 另一种是无水CuCl粉末,其在空气中放置短时间内易发生水解和氧化. 试验结果表明,该XPS 原位转移仓对不同类型的空气敏感样品的无空气转移均可以提供更便捷有效的密封保护. 目前,XPS原位转移仓已在多个科研单位的实验室推广使用,支撑应用涉及吸附与催化、能源环境等研究领域.1 试验部分1.1 XPS原位转移仓的研制基于本实验室ESCALAB 250Xi型多功能光电子能谱仪器(Thermofisher 公司)的特点,研究人员设计了XPS原位转移仓. 为兼顾各个部件强度、精度与轻量化的要求,所有部件均采用钛合金材料.该装置从整体结构上分为样品台、密封罩和紧固挡板三个部件,如图1(a)~(c)所示. 在密封罩内部通过单向密封设计[图1(e)]使得XPS原位转移仓实现正负压一体,实际操作中可通过调节密封罩上的螺帽完成两种模式任意切换. 同时,从图1(e)中可以直观看到,密封罩与样品台之间通过O圈密封,利用带有螺钉的紧固挡板将二者紧密固定. 此外,为确保样品台与密封罩对接方位正确,本设计使用定向槽定位样品台与密封罩位置,保证XPS原位转移仓顺利传接到仪器进样室.XPS原位转移仓使用的具体流程:在手套箱中将空气敏感样品粘贴至样品台上,利用紧固挡板使样品台和密封罩固定在一起,通过调节密封罩上的螺帽将样品所在区域密封为正压惰性气氛(压强为300 Pa、环境气氛与手套箱内相同)或者负压真空状态,其整体装配实物图如图1(d)所示. 该转移仓结构小巧,整体尺寸仅52 mm×58 mm×60 mm,可直接放入手套箱小过渡舱传递. 由于转移仓尺寸小,其第 1 期章小余,等:正负压一体式无空气X射线光电子能谱原位转移仓的开发及研制31原料成本大大缩减,整体造价不高. 转移仓送至能谱仪进样室后,配合样品停放台与进样杆的同时双向对接,将转移仓整体固定在进样室内,如图1(f )所示. 此时关闭进样室舱门开始抽真空,当样品台与密封罩内外压强平衡后密封罩自动解除真空密封,但仍然处于O 圈密闭状态. 等待进样室真空抽至1×10−4Pa 后,使用能谱仪进样室的样品停放台摘除脱离的密封罩[如图1(g )所示],待真空抽至1×10−6Pa ,即可将样品送入分析室进行XPS 测试.整个试验过程操作便捷,实现了样品从手套箱转移至能谱仪内不接触大气环境.1.2 试验过程1.2.1 样品准备及转移试验所用手套箱是布劳恩惰性气体系统(上海)有限公司生产,型号为MB200MOD (1500/780)NAC ;金属Li 片购自中能锂业,纯度99.9%;CuCl 购自ALFA 公司,纯度99.999%.金属Li 片的制备及转移:将XPS 原位转移仓整体通过手套箱过渡舱送入手套箱中,剪取金属Li 片用双面胶带固定于样品台上,分别采用正压、负压两种密封模式将XPS 原位转移仓整体从手套箱中取出,分别在空气中放置0、2、4、8、18、24、48、72 h 后送入能谱仪内,进行XPS 测试.CuCl 粉末的制备及转移:在手套箱中将CuCl 粉末压片[21],使用上述同样的制备方法,将XPS 原位转移仓整体在空气中分别放置0、7、24、72 h 后送入能谱仪内,进行XPS 测试.1.2.2 样品转移方式介绍样品在手套箱中粘贴完成后,分别采用三种方式将其送入能谱仪. 第一种方式是在手套箱内使用标准样品台粘贴样品,将其装入自封袋密封,待能谱仪进样室舱门打开后,即刻打开封口袋送入仪器中开始抽真空等待测试,整个转移过程中样品暴露空气约15 s. 第二种方式是使用XPS 原位转移仓负压密封模式转移样品,具体操作步骤:利用紧固挡板将样品台和密封罩固定在一起,逆时针(OPEN )旋动螺帽至顶部,放入手套箱过渡舱并将其抽为真空,此过程中样品所在区域也抽至负压. 取出整体装置后再顺时针(CLOSE )旋动螺帽至底部,将样品所在区域进一步锁死密封. 样品在负压环境中转移至XPS 实验室,拆卸掉紧固挡板,随即送入能谱仪进样室内. 第三种方式是使用XPS 原位转移仓正压密封模式转移样品,具体操作步骤:利用紧固挡板将样品台和密封罩固定在一起,顺时针(CLOSE )旋螺帽抽气管限位板单向密封柱密封罩主体O 圈样品台紧固挡板(e) 密封罩对接停放台机械手样品台对接进样杆(a)(b)(c)(d)(g)图1 正负压一体式无空气XPS 原位转移仓系统装置(a )样品台,(b )密封罩,(c )紧固挡板,(d )整体装配实物图,(e )整体装置分解示意图,(f )样品台与密封罩在进样室内对接完成,(g )样品台与密封罩在进样室内分离Fig. 1 System device of inert-gas/vacuum sealing air-free XPS in-situ transfer module32分析测试技术与仪器第 29 卷动螺帽至底部,此时样品所在区域密封为正压惰性气氛. 直至样品转移至XPS 实验室,再使用配套真空抽气系统(如图2所示),通过抽气管将样品所在区域迅速抽为负压,拆卸掉紧固挡板,随即送入能谱仪进样室内.图2 能谱仪实验室内配套真空抽气系统Fig. 2 Vacuum pumping system in XPSlaboratory1.2.3 XPS 分析测试试验所用仪器为Thermo Fisher Scientific 公司的ESCALAB 250Xi 型多功能X 射线光电子能谱仪,仪器分析室基础真空为1×10−7Pa ,X 射线激发源为单色化Al 靶(Alk α,1 486.6 eV ),功率150 W ,高分辨谱图在30 eV 的通能及0.05 eV 的步长等测试条件下获得,并以烃类碳C 1s 为284.8 eV 的结合能为能量标准进行荷电校正.2 结果与讨论2.1 测试结果分析为了验证XPS 原位转移仓的密封性能,本文做了一系列的对照试验,选取空气敏感的金属Li 片和CuCl 粉末样品进行XPS 测试,分别采用上述三种方式转移样品,并考察了XPS 原位转移仓密封状态下在空气中放置不同时间后对样品测试结果的影响.2.1.1 负压密封模式下XPS 原位转移仓对金属Li片的密封时效性验证将金属Li 片通过两种(标准和负压密封)方式转移并在空气中放置不同时间,对这一系列样品进行XPS 测试,Li 1s 和C 1s 高分辨谱图结果如图3(a )(b )所示,试验所测得的Li 1s 半峰宽值如表1所列. 根据XPS 结果分析,金属Li 片采用标准样品台进样(封口袋密封),短暂暴露空气约15 s ,此时Li 1s 的半峰宽为1.62 eV. 而采用XPS 原位转移仓负压密封模式转移样品时,装置整体放置空气18 h 内,Li 1s 的半峰宽基本保持为(1.35±0.03) eV. 放置空气24 h 后,Li 1s 的半峰宽增加到与暴露空气15 s 的金属Li 片一样,说明此时原位转移仓的密封性能衰减,金属Li 片与渗入内部的空气发生反应生成新物质导致Li 1s 半峰宽变宽. 由图3(b )中C 1s 高分辨谱图分析,结合能位于284.82 eV 的峰归属为C-C/污染C ,位于286.23 eV 的峰归属为C-OH/C-O-CBinding energy/eVI n t e n s i t y /a .u .Li 1s半峰宽增大暴露 15 s密封放置 24 h 密封放置 18 h 密封放置 8 h 密封放置 4 h 密封放置 0 h6058565452Binding energy/eVI n t e n s i t y /a .u .C 1s(a)(b)暴露 1 min 暴露 15 s 密封放置 24 h 密封放置 18 h 密封放置 0 h292290288284282286280图3 金属Li 片通过两种(标准和负压密封)方式转移并在空气中放置不同时间的(a )Li 1s 和(b )C 1s 高分辨谱图Fig. 3 High-resolution spectra of (a) Li 1s and (b) C 1s of Li sheet samples transferred by two methods (standard andvacuum sealings) and placed in air for different times第 1 期章小余,等:正负压一体式无空气X 射线光电子能谱原位转移仓的开发及研制33键,位于288.61~289.72 eV的峰归属为HCO3−/CO32−中的C[22]. 我们从C 1s的XPS谱图可以直观的看到,与空气短暂接触后,样品表面瞬间生成新的结构,随着暴露时间增加到1 min,副反应产物大量增加(HCO3−/CO32−). 而XPS原位转移仓负压密封模式下在空气中放置18 h内,C结构基本不变,在空气中放置24 h后,C结构只有微小变化. 因此根据试验结果分析,对于空气极其敏感的材料,在负压密封模式下,建议XPS原位转移仓在空气中放置时间不要超过18 h. 这种模式适合对空气极其敏感样品的短距离转移.表 1 通过两种(标准和负压密封)方式转移并在空气中放置不同时间的Li 1s的半峰宽Table 1 Full width at half maxima (FWHM) of Li 1stransferred by two methods (standard and vacuum sealings) and placed in air for different times样品说明进样方式半峰宽/eV密封放置0 h XPS原位转移仓负压密封模式转移1.38密封放置2 h同上 1.39密封放置4 h同上 1.36密封放置8 h同上 1.32密封放置18 h同上 1.32密封放置24 h同上 1.62暴露15 s标准样品台进样(封口袋密封)1.622.1.2 正压密封模式下原位转移仓对金属Li片的密封时效性验证将金属Li片通过两种(标准和正压密封)方式转移并在空气中放置不同时间,对这一系列样品进行XPS测试,Li 1s高分辨谱图结果如图4所示,所测得的Li 1s半峰宽值如表2所列. 根据XPS结果分析,XPS原位转移仓正压密封后,在空气中放置72 h内,Li 1s半峰宽基本保持为(1.38±0.04) eV,说明有明显的密封效果,金属Li片仍然保持原有化学状态. 所以对于空气极其敏感的材料,在正压密封模式下,可至少在72 h内保持样品表面不发生化学态变化. 这种模式适合长时间远距离(可全国范围内)转移空气敏感样品.2.1.3 负压密封模式下XPS原位转移仓对空气敏感样品CuCl的密封时效性验证除了金属Li片样品,本文还继续考察XPS原位转移仓对空气敏感样品CuCl的密封时效性. 图5为CuCl粉末通过两种(标准和负压密封)方式转移并在空气中放置不同时间的Cu 2p高分辨谱图. XPS谱图中结合能[22]位于932.32 eV的峰归属为Cu+的Cu 2p3/2,位于935.25 eV的峰归属为Cu2+的Cu 2p3/2,此外,XPS谱图中位于940.00~947.50 eV 处的峰为Cu2+的震激伴峰,这些震激伴峰被认为是表 2 通过两种(标准和正压密封)方式转移并在空气中放置不同时间的Li 1s的半峰宽Table 2 FWHM of Li 1s transferred by two methods(standard and inert gas sealings) and placed in air fordifferent times样品说明进样方式半峰宽/eV 密封放置0 h XPS原位转移仓正压密封模式转移1.42密封放置2 h同上 1.35密封放置4 h同上 1.35密封放置8 h同上 1.34密封放置18 h同上 1.38密封放置24 h同上 1.39密封放置48 h同上 1.42密封放置72 h同上 1.38暴露15 s标准样品台进样(封口袋密封)1.62Binding energy/eVIntensity/a.u.Li 1s半峰宽比正压密封的宽半峰宽=1.62 eV半峰宽=1.38 eV暴露 15 s密封放置 72 h密封放置 48 h密封放置 24 h密封放置 18 h密封放置 0 h605856545250图4 金属Li片通过两种(标准和正压密封)方式转移并在空气中放置不同时间的Li 1s高分辨谱图Fig. 4 High-resolution spectra of Li 1s on Li sheet samples transferred by two methods (standard and inert gas sealings) and placed in air for different times34分析测试技术与仪器第 29 卷价壳层电子向激发态跃迁的终态效应所产生[23],而在Cu +和Cu 0中则观察不到.根据XPS 结果分析,CuCl 在XPS 原位转移仓保护(负压密封)下,即使放置空气中72 h ,测得的Cu 2p 高分辨能谱图显示只有Cu +存在,说明CuCl 并未被氧化. 若无XPS 原位转移仓保护,CuCl 粉末放置空气中3 min 就发生了比较明显的氧化,从测得的Cu 2p 高分辨能谱图能够直观的看到Cu 2+及其震激伴峰的存在,并且随着放置时间增加到40 min ,其氧化程度也大大增加. 因此,对于空气敏感的无机材料、纳米催化剂和钙钛矿材料等,采用负压密封模式转移就可至少在72 h 内保持样品表面不发生化学态变化.3 结论本工作中自主研制的正负压一体式无空气XPS原位转移仓在空气敏感样品转移过程中可以有效隔绝空气,从而获得样品最真实的表面化学结构.试验者可根据样品情况和实验室条件选择转移模式,并在密封有效时间内将样品从实验室转移至能谱仪中完成测试. 综上所述,该XPS 原位转移仓是一种设计小巧、操作简便、密封性能优异、成本较低的样品无水无氧转移装置,因此非常适合广泛开放给有需求的试验者使用. 在原位和准原位表征技术被广泛用于助力新材料发展的现阶段,希望该设计理念能对仪器功能的开发和更多准原位表征测试的扩展提供一些启示.参考文献:黄惠忠. 论表面分析及其在材料研究中的应用[M ].北京: 科学技术文献出版社, 2002: 16-18.[ 1 ]杨文超, 刘殿方, 高欣, 等. X 射线光电子能谱应用综述[J ]. 中国口岸科学技术,2022,4(2):30-37.[YANG Wenchao, LIU Dianfang, GAO Xin, et al.TheapplicationofX -rayphotoelectronspectroscopy [J ]. China Port Science and Technology ,2022,4 (2):30-37.][ 2 ]郭沁林. X 射线光电子能谱[J ]. 物理,2007,36(5):405-410. [GUO Qinlin. X -ray photoelectron spectro-scopy [J ]. Physics ,2007,36 (5):405-410.][ 3 ]Malmgren S, Ciosek K, Lindblad R, et al. Con-sequences of air exposure on the lithiated graphite SEI [J ]. Electrochimica Acta ,2013,105 :83-91.[ 4 ]Zhang Y H, Chen S M, Chen Y, et al. Functional poly-ethylene glycol-based solid electrolytes with enhanced interfacial compatibility for room-temperature lithium metal batteries [J ]. Materials Chemistry Frontiers ,2021,5 (9):3681-3691.[ 5 ]周逸凡, 杨慕紫, 佘峰权, 等. X 射线光电子能谱在固态锂离子电池界面研究中的应用[J ]. 物理学报,2021,70(17):178801. [ZHOU Yifan, YANG Muzi,SHE Fengquan, et al. Application of X -ray photoelec-tron spectroscopy to study interfaces for solid-state lithium ion battery [J ]. Acta Physica Sinica ,2021,70(17):178801.][ 6 ]Huang J J, Song Y Y, Ma D D, et al. The effect of thesupport on the surface composition of PtCu alloy nanocatalysts: in situ XPS and HS-LEIS studies [J ].Chinese Journal of Catalysis ,2017,38 (7):1229-1236.[ 7 ]Koley P, Shit S C, Sabri Y M, et al. Looking into moreeyes combining in situ spectroscopy in catalytic bio-fuel upgradation with composition-graded Ag-Co core-shell nanoalloys [J ]. ACS Sustainable Chemistry &Engineering ,2021,9 (10):3750-3767.[ 8 ]Opitz A K, Nenning A, Rameshan C, et al. Enhancingelectrochemical water-splitting kinetics by polarization-driven formation of near-surface iron(0): an in situ XPS study on perovskite-type electrodes [J ]. Ange-wandte Chemie (International Ed in English),2015,54(9):2628-2632.[ 9 ]Czekaj I, Loviat F, Raimondi F, et al. Characterization[ 10 ]Binding energy/eVI n t e n s i t y /a .u .Cu 2pCu +Cu 2+暴露 3 min暴露 40 min 密封放置 7 h 密封放置 72 h 密封放置 24 h密封放置 0 h960950945935925955940930920图5 CuCl 粉末通过两种(标准和负压密封)方式转移并在空气中放置不同时间的Cu 2p 高分辨谱图Fig. 5 High-resolution spectra of Cu 2p on CuCl powder samples transferred by two methods (standard and vacuumsealings) and placed in air for different times第 1 期章小余,等:正负压一体式无空气X 射线光电子能谱原位转移仓的开发及研制35of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X -ray photoelectron spectroscopy (XPS)[J ]. Applied Cata-lysis A:General ,2007,329 :68-78.Rutkowski M M, McNicholas K M, Zeng Z Q, et al.Design of an ultrahigh vacuum transfer mechanism to interconnect an oxide molecular beam epitaxy growth chamber and an X -ray photoemission spectroscopy analysis system [J ]. Review of Scientific Instruments ,2013,84 (6):065105.[ 11 ]伊晓东, 郭建平, 孙海珍, 等. X 射线光电子能谱仪样品前处理装置的设计及应用[J ]. 分析仪器,2008(5):8-11. [YI Xiaodong, GUO Jianping, SUN Haizhen, et al. Design of a sample pretreatment device for X -ray photoelectron spectrometer [J ]. Analytical Instrumentation ,2008 (5):8-11.][ 12 ]Aurbach D, Weissman I, Schechter A, et al. X -ray pho-toelectron spectroscopy studies of lithium surfaces pre-pared in several important electrolyte solutions. A comparison with previous studies by Fourier trans-form infrared spectroscopy [J ]. Langmuir ,1996,12(16):3991-4007.[ 13 ]Światowska-Mrowiecka J, Maurice V, Zanna S, et al.XPS study of Li ion intercalation in V 2O 5 thin films prepared by thermal oxidation of vanadium metal [J ].Electrochimica Acta ,2007,52 (18):5644-5653.[ 14 ]Weingarth D, Foelske-Schmitz A, Wokaun A, et al. Insitu electrochemical XPS study of the Pt/[BF 4]system [J ]. Electrochemistry Communications ,2011,13 (6):619-622.[ 15 ]Schneider J D, Agocs D B, Prieto A L. Design of asample transfer holder to enable air-free X -ray photo-electron spectroscopy [J ]. Chemistry of Materials ,2020,32 (19):8091-8096.[ 16 ]Karamurzov B S, Kochur A G, Misakova L B, et al.Calculation of the pure surface composition of the bin-ary alloy according to XPS data obtained after the al-loy surface contact with air [J ]. Journal of Structural Chemistry ,2015,56 (3):576-581.[ 17 ]章小余, 赵志娟. 一种半原位XPS 样品转移装置: 中国, 201620925237.5[P ]. 2017-02-15.[ 18 ]章小余, 袁震, 赵志娟. 一种半原位X 射线光电子能谱分析仪的样品转移装置: 中国, 201720056623.X [P ]. 2017-12-08.[ 19 ]袁震, 章小余, 赵志娟. 一种样品转移装置及转移方法: 中国, 2011203822.1[P ]. 2022-03-01.[ 20 ]刘芬, 赵志娟, 邱丽美, 等. XPS 分析固体粉末时的样品制备法研究[J ]. 分析测试技术与仪器,2007,13(2):107-109. [LIU Fen, ZHAO Zhijuan, QIU Limei, et al. Study of sample preparation method for XPS analysis of powdered samples [J ]. Analysis and Testing Technology and Instruments ,2007,13 (2):107-109.][ 21 ]Wagner C D, Riggs W M, Davis L E, et al. Handbookof X -ray photoelectron spectroscopy [M ]. Eden Prair-ie, Minnesota, 1978.[ 22 ]Watts J F, Wolstenholme J. 表面分析(XPS 和AES)引论[M ]. 吴正龙, 译. 上海: 华东理工大学出版社,2008.[ 23 ]36分析测试技术与仪器第 29 卷。
第36卷第4期2018年8月低温与特气.Low Temperature and Specialty GasesVol. 36,No. 4Apr.,2〇18•应用技术•气体稳流阀在火焰离子化气相色谱中的应用罗旭,孙晓W(中昊光明化工研究设计院有限公司,辽宁大连116031)摘要:稳流阀,具有稳定流速的特性。
阐述了气体稳流阀应用在火焰离子化气相色谱仪器中的作用与必要性。
关键词:稳流阀;火焰离子;气相色谱中图分类号:TQ117 文献标志码:A 文章编号:1007-7804(2018)04-0049-04d oi:10. 3969/j. issn. 1007-7804. 2018. 04. 013Application of Gas Steady Flow Valve in Fid Gas ChromatographyLU O X u,SUN X ia o li(Zhonghao Guangming Research Design Institute of Chemical Industry C o.,L td.,Dalian 116031,China)A b stract:Steady flow valve,characteristic o f steady flow rate. This paper discusses the function and necessityflow valve used in F ID gas chromatography.K ey words :steady flow valve ;FID ;gas chromatography随着科技的发展,色谱仪器技术也在突飞猛进,从早期的国外技术垄断到现在的自主设计生产,科 研人员付出了诸多的汗水。
现如今,色谱仪器产品 种类越来越多,其中气相色谱尤以使用方便、价格相 对低廉等特点而被广泛应用。
在应用范围方面,气 相色谱主要可以分为通用型和专用型两大类。
S¯a dhan¯a V ol.27,Part5,October2002,pp.569–575.©Printed in IndiaMeltflow characteristics in gas-assisted laser cuttingB TIRUMALA RAO and A K NATHCentre for Advanced Technology,Indore452013,Indiaemail:trao@cat.ernet.inMS received24May2001;revised28December2001Abstract.We present a study on laser cutting of mild steel with oxygen asan assist gas.We correlate the cut surface quality with the meltfilm thickness.We estimate the optimum pressure required for melt ejection under laminarflowregime.The thickness of meltfilm inside the kerf is estimated using mass balanceand the shear force acting on the cutting front assuming meltflow profile as linear.The dependence of meltfilm thickness on gas pressure,cutting velocity and workpiece thickness is estimated and compared with experimental results.ser cutting;gasflow;shear stress;meltfilm thickness.1.IntroductionCarbon dioxide(CO2)laser cutting of thick mild steel with oxygen is one of the evolving industrial applications.In this process the cut surface quality is very important.The basic parameters in laser cutting are cutting speed,kerf width,laser power,chemistry of gas, nozzle exit pressure,nozzle design,workpiece thickness and surface quality.In oxygen assist laser cutting,the gas jet plays two roles,one is to generate additional thermal energy during formation of FeO and other oxides.This additional energy enhances the process parameters like cutting speed and thickness of the workpiece.The second role is to supply the shear force to the gas/liquid boundary to eject the molten metal formed during the cutting process. In laser cutting the major process is the instantaneous melt ejection,which is achieved by selecting appropriate operating window of the cutting speed,gas pressure,laser power,and stand-off distance etc.,The cut surface quality is influenced by the meltfilm thickness developed inside the kerf during the process,it will be poor for larger melt thickness.Therefore it is important to understand how various experimental parameters influence the meltfilm thickness.Although much work has been done in this direction the phenomenon of melt ejection is poorly defined due to its complex nature.The meltfilm thickness is dependent on the FeO formation,since FeO melt has lower viscosity compared to the Fe melt.The fraction of FeO formation reduces with increasing cutting speed up to a certain limit and then reaches a steady value as reported by Kaplan(1996).Yilbas&Kar(1998)reported a similar trend in the variation of meltfilm thickness with assist-gas velocity.A list of symbols is given at the end of the paper569570B Tirumala Rao and A K NathThe present study is confined to gas-assisted cutting with the convergent nozzle and very small stand-off distance.The work does not deal with the thermodynamics of melt formation or heatflow into the work piece.The objective of the present work is to estimate the optimum pressure required for melt ejection under laminarflow regime,using a uniformflow over a flat plate(Landau&Lifshitz1987;Kumar1988).Also the meltfilm thickness inside the kerf during the process is estimated by the analytical model taking into account the mass balance of molten metal coupled with shear force required to drag the moltenfilm.The dependence of the gas pressure,cutting speed and workpiece thickness on meltfilm thickness is modelled and compared with the experimental results.2.Model descriptionThe laser-cutting setup is shown in thefigure1.A fraction of the gas coming out of the converging nozzle exit enters into the kerf with a constant velocity(Shapiro1953;Liepmann &Roshko1957)independent of the stagnation pressure provided that the nozzle exit is maintained at the critical state.The nozzle exit is said to be in critical state,when the ratio of ambient to stagnation pressure is less than0.5283(Shapiro1953;Liepmann&Roshko1957). In the critical state the exit gas velocity would be301m/s for O2at stagnation temperature of 300K(Shapiro1953;Liepmann&Roshko1957).We assume that nozzle stand-off distance is very small,the melt surface isflat,the kerf width is very large(>500µm)compared to the gaseous boundary layer(∼50µm)(Kumar1988;Heidenreich et al1996)and the variation of the gas density is very small.Under these assumptions the boundary layer theoryflow over Laser beamBeam folderPressure gauge Focussing lensCutinggasinletNozzleWork pieceCut kerf ser cutting setup.Meltflow characteristics in gas-assisted laser cutting571 aflat plate can be applied to estimate the distance after which theflow separation takes place by calculating the critical Reynolds number(Landau&Lifshitz1987;Kumar1988),Re z=UZρ/µ.(1) This is because a laminar layer tends to separate off the surface more readily than a turbulent layer(Kumar1988).When the value of Re z exceeds3.2×105the gasflow suffers a change from laminar to turbulent;this is called the separation point(Landau&Lifshitz1987;Kumar 1988;Heidenreich et al1996).At this separation point the shear stress exerting on the melt is significantly reduced due to wake formation,which results in inefficient melt ejection and thicker molten layer.Also in this region the diffusion of oxygen in Fe changes due to turbulence,consequently this develops poor cut quality.To achieve more or less uniform cut quality the gasflow in the kerf should be laminar in ing the critical Reynolds number at the separation and relations amongst the critical gas pressure,density and temperature (Shapiro1953;Liepmann&Roshko1957)the following relation gives the depth of the cut after which the gasflow tends to separate with respect to the stagnation gas pressure.Z=22.5/P0,(2)From this relation we canfind the nozzle pressure for a given thickness d of the sheet,so theflow should be laminar throughout the depth,for which Z=d.The above relationship is valid closely for the larger kerf width,since the length of the laminar gasflow inside the kerf is dependent on the kerf width(Heidenreich et al1996).If the kerf width is small then the value of Z reduces significantly.The shear force acting on the meltfilm at the gas/liquid interface is(Vicanek&Simon 1987;Kumar1988;Yilbas&Kar1998),τ=0.332(ρU2)/¯Re1/2z.(3)The present model balances this shear force with the melt viscous forces.Since the thickness of the meltfilm is very small(Yilbas&Kar1998),we assume that melt velocity profile is linear(figure2).The maximum melt velocity u max is at gas/melt interface and zero melt U = 0U = UmaxMolten metal Molten metal-gas boundarySolid-molten metalboundaryZ Figure2.Melt velocity profile.572B Tirumala Rao and A K Nathvelocity is at melt/solid interface.If u avg is the melt average velocity,which is u max/2,and melt is ejected due to the shear force given by the gas jet,thenτ=µm(u max/ ).From this we get u avg=τ /2µm.(4) Mass balance criteria:the amount of molten mass in per unit time=ρm v c d w,the amount of molten mass out per unit time=ρm u avg w.In the steady state:molten mass in per unit time=molten mass out per unit time.Also by substituting melt average velocity(4)in the equation we get the meltfilm thickness =(2µm v c Z/τ)1/2.(5) This relation gives the dependence of meltfilm thickness on gas pressure,cutting speed and workpiece thickness.3.Experimental results and discussionsReynolds number increases at any given depth with gas pressure,(1).The extent of lami-narflow in the kerf is dependent on stagnation gas pressure in the nozzle.Maintaining the gasflow in the laminar regime,the operating range of pressure can be large in thin sheet cutting.Figure3shows the stagnation gas pressure that can sustain laminar gasflow in the entire thickness of the workpiece under the assumption that the nozzle stand-off distance is0.5mm and the kerf width is about∼1mm.Since the online measurement of meltfilm thickness is beyond the scope of the present experimental study,we try to estimate it using the striation wavelength.Since striation wavelength is decided by surface tension effects of the melt,oxidation front propagation speed and laser beam diameter at the metal sur-face,besides the cut speed and gasflow velocity,the estimated meltfilm thickness from the striation wavelength is expected to be on the higher side.Figure4shows the varia-tion of meltfilm thickness with the thickness of the workpiece at constant cutting velocity and different stagnation gas pressures.Operating at higher gas pressure results in minimum meltfilm thickness sustained on the cut surface.Figure5shows the variation of meltfilm thickness with workpiece thickness for different cutting velocities at constant gas pressure. When operated at higher cutting velocity thick meltfilm sustains as very coarse striations on the cut surface.Experimental values are also plotted on the samefigures.While the experimental and theoretical trends of variations match well,the discrepancy in actual val-ues could be due to the overestimation of experimental values of thickness as mentioned above.Also the impact of cutting speed on the meltfilm thickness(at same laser power600W and gas pressure1bar=105N/m2)can be seen infigure6.Figures6a and b depict the laser cut surfaces of5mm thick mild steel plate when cutting is done at500mm/min and 1000mm/min speeds respectively.The one with higher cutting speed has coarse striations indicating the existence of thicker meltfilm during the cutting.Figure7shows the laser cut surface of5mm thick mild steel plate with gas pressure of3bar and cutting speed of 500mm/min.Meltflow characteristics in gas-assisted laser cutting573Figure3.Stagnation gas pressure vs work-piece thickness.Figure4.Meltfilm thickness variationwith gas pressure.Figure5.Meltfilm thickness variationwith cutting velocity(a)(b)Figure ser cut surface of MS(5mm):(a)speed=500mm/min,pressure=1bar;(b)speed=1000mm/min,pressure=1bar.574B Tirumala Rao and A K Nathser cut surface of MS(5mm)(a)speed=500mm/min,pressure=1bar.The impact of change in gas pressure on the meltfilm thickness is not as much as that of cutting speed.The same is evident formfigure4(change in gas pressure)andfigure5(change in cutting velocity),and also from the cut surfaces shown infigures6a and7(change in gas pressure),andfigures6a and b(change in cutting velocity.).For5mm thick mild steel plates at600W laser power and one bar gas pressure the maximum cutting speed is1300mm/min, and when operated at3bar gas pressure the optimum cutting speed is1500mm/min with good cut edge quality.Beyond these speeds the bottom part of the cut is attached with the dross. Figure8a shows the laser cut surface of10mm thick mild steel plate when cutting is done at1.5bar.It is found that during cutting at higher gas pressure(3bar)the lower part of the cut surface is badly damaged as shown infigure8b.This could be due to turbulent gasflow as explained earlier.4.ConclusionsTo achieve minimum meltfilm thickness on the cut surface laser cutting should be performed at optimum cutting velocity and maximum gas pressure such that laminar gasflow is sustained in the entire kerf depth.For a sufficiently large kerf width in thin sheet cutting the operating range of gas pressure is more than that in thick sheet cutting,as laminarflow can be maintained throughout the depth of the cut in thin sheets.In other words,thin sheets can be cut with an optimum gas pressure and a smaller kerf width with laminar gasflow.To cut thick sheets,the focal point of the beam should be inside the workpiece so that it provides larger kerf width,which allows theflow to be laminar up to larger depths.To achieve (a)(b)ser cut surface of MS(10mm):(a)speed=300mm/min,pressure=1bar;(b)speed=300mm/min,pressure=3bar.Meltflow characteristics in gas-assisted laser cutting575 better cut quality in thick plates(>10mm),the operating pressure should be less than2bar so that laminar gasflow is sustained in the entire depth.The meltfilm thickness,which decides the roughness of the cut surface,is influenced to a greater extent by the change in cutting velocity than the variation in the cutting nozzle gas pressure.The authors would like to thank R Sridhar and C H Prem Singh for their kind assistance in the experiments.List of symbolsd thickness of the work piece(m);P0stagnation gas pressure(bar,1bar=105N/m2);s nozzle stand-off distance(m);U gas jet velocity(m/s);u avg average melt velocity(m/s);v c cutting velocity(m/s);w kerf width(m);Z depth from nozzle exit(mm);ρdensity of the gas(kg/m3);ρm density of molten metal(kg/m3);µgas(O2)viscosity(∼1.7×10−5kg/ms);µm melt viscosity(∼4×10−3kg/ms);τshear stress of the gas(N/m2);meltfilm thickness(m).ReferencesHeidenreich B,Juptner W,Sepold G1996Fundamental investigation of the burn-out phenomenon of laser cut sers Eng.5:1–10Kaplan A F H1996An analytical model of metal cutting with a laser beam.J.Appl.Phys.79:2198–2207Kumar K L1988Engineeringfluid mechanics4th edn(New Delhi:Eurasia)Landau L D,Lifshitz E M1987Fluid mechanics2nd rev.edn(Oxford:Butterworth-Heinemann) Liepmann H W,Roshko A1957Elements of gas dynamics(New York:John Wiley&Sons) Shapiro H A1953The dynamics&thermodynamics of compressiblefluidflow(New York:John Wiley &Sons)Vicanek M,Simon G1987Momentum and heat transfer of an inert gas jet to the melt in laser cutting.J.Phys.D20:1191–1196Yilbas B S,Kar A1998Thermal and efficiency analysis of CO2laser cutting sers Eng.29:17–32。