金域传奇产品手册终稿(EF已调整)
- 格式:pdf
- 大小:18.67 MB
- 文档页数:171


******有限公司****** CO.,LTD质量手册Quality Manual依据YY/T0287-2017 idt ISO13485:2016和ISO9001:2015标准编制文件编号: *** - QM版次:A/0生效日期: 2020年**月**日分发号:编制人:日期:2020年**月**日审核人:日期:2020年**月**日批准人:日期:2020年**月**日内部文件注意保存颁布令为了满足市场的需求和顾客的要求,适应市场的竞争,不断提高我公司的质量管理水平,依据YY/T 0287-2017 idt ISO 13485:2016《医疗器械质量管理体系用于法规的要求》和ISO9001:2015《质量管理体系要求》制定了本《质量手册》,经审定本《质量手册》符合国家有关政策、法律法规和本公司实际情况,现正式批准发布,从2020年2月23日开始实施。
本《质量手册》是公司质量管理体系的纲领性文件,规定了影响产品的各种活动的程序要求及相关部门的质量职责,是公司各部门和全体干部员工开展质量管理活动的依据和行动准则;对外是公司质量管理的承诺,是向客户证明产品质量符合法律法规要求的保证能力、开展质量管理活动,并保持其持续有效性。
现予以批准颁发,公司全体员工必须认真学习、理解质量方针,并将《质量手册》所规定的各项程序要求贯彻落实到日常工作中,以实现公司质量目标及经济效益。
特此发布。
总经理:2020年**月**日管理者代表任命书兹任命 *** 先生为管理者代表。
负责按ISO 13485:2016和ISO9001:2015标准的要求,确保公司质量管理体系所需的过程得到建立、实施和保持;及时向公司总经理报告质量管理体系的业绩和任何改进的需求;确保在公司内部提高满足法规要求和顾客要求的意识;并就质量管理体系的有关事项与外部联络和沟通。
希望公司所有员工服从协调,共同履行质量职能,确保质量管理体系有效运行。
总经理:2020年**月**日目录0 引言 (5)0.1公司简介 (5)0.2质量手册的管理 (6)0.3 有关不适用条款的说明 (8)0.4 生产和服务流程图 (9)0.5质量方针和质量目标 (10)0.6 组织机构图 (11)0.7 质量管理体系部门目标 (12)1 范围 (13)2 引用标准 (13)3 术语和定义 (13)4 质量管理体系 (15)4.1 总要求 (15)4.2文件要求 (16)5 管理职责 (18)5.1 管理承诺 (18)5.2 以顾客/法规为关注焦点 (19)5.3质量方针 (19)5.4 策划 (20)5.5 职责、权限和沟通 (21)5.6 管理评审 (25)6 资源管理 (26)6.1 资源的提供 (26)6.2 人力资源 (26)6.3 基础设施 (26)6.4 工作环境 (26)7 产品实现 (27)7.1 产品实现的策划 (27)7.2 与顾客有关的过程 (28)7.3 设计和开发 (28)7.4 采购 (31)7.5 生产和服务提供 (32)7.6 监视和测量装置的控制 (35)8 测量、分析和改进 (36)8.1 总则 (36)8.2 监视和测量 (36)8.3 不合格品控制 (38)8.4 数据分析 (39)8.5 改进 (39)程序文件清单 (41)0 引言0.1公司简介******有限公司,位于深圳市龙岗区平湖社区,毗邻华南城,是一家专注于医用防护产品的医疗器械公司。
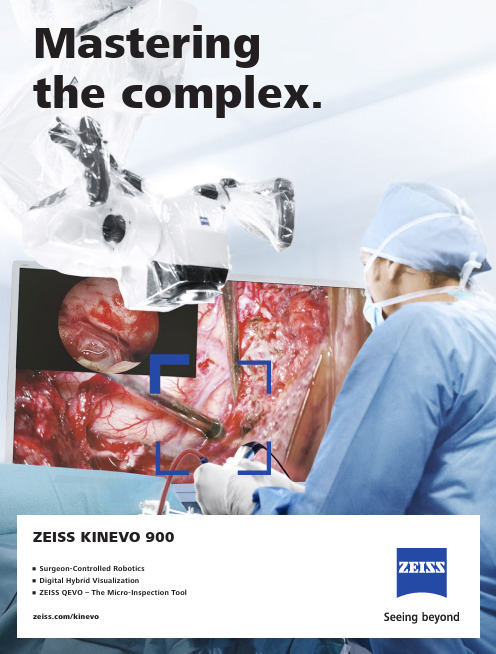
/kinevoZEISS KINEVO 900Mastering the complex.• Surgeon-Controlled Robotics • Digital Hybrid Visualization • ZEISS QEVO – The Micro-Inspection ToolZEISS KINEVO 900The Robotic Visualization SystemJust like you, we love challenging the status quo.The result? Over 100 innovations to perfect the already acclaimed surgical visualization platform. KINEVO® 900 from ZEISS is designed to deliver more functionalities than any surgical microscope today. ZEISS KINEVO 900 combines digital and optical visualization modalities, offers a unique Micro-Inspection Tool and will impress you with its Surgeon-Controlled Robotics. All to enable you to gain greater certainty in a virtually disruption-free workflow.Designed to meet real needs. To make a real difference!A lot more. And, a lot less too.When treating complex vascular conditions, you typically work at high magnification. Even the slightest vibrations can cause disruptions. And constant manual repositioning to better visualize structures or precisely approach deep-seated lesions can become extremely tedious. Not anymore! ZEISS KINEVO 900 delivers a lot more positioning precision with a lot less effort.PointLockSurgeon-Controlled Robotics adds a complete new level of ease to precise positioning. Imagine being able to focus and move around a structure to visualize the targeted anatomy – reducing any manual hassle. In addition, PointLock enables you to do a KeyHole movement to observe a larger area inside a cavity – a particular benefit in areas with narrow access. Simply put:Focus. Activate. Swivel.Active vibration dampingYou know the problems that can be created by the tiniest vibrations. The active damping provided by ZEISS KINEVO 900 minimizes collateral system vibrations, ensuring rock-solid stability. Enabling you to completely, and steadily, focus on what matters most: your treatment.Focus Activate Swivel5When you need it. Where you need it.The new navigation interface of ZEISS KINEVO 900 is designed to work in concert with your navigation device. When you require precise repositioning to reexamine previously visualized structures or when you need to align with a pre-mapped trajectory, making use of all six axes, the Robotic Visualization System ® delivers precise positioning at the push of a button. Putting you exactly where you need to be – when you need to be there.PositionMemoryWhen working on a tumor case, you may already have identified regions of concern where you want to protect the functional structure. After storing these in PositionMemory , you can come back and visualize them at the exact same magnification, working distance and focus – without losing time for manual repositioning. In a nutshell: Save. Move. Recall.Image-guided surgeryApproaching deep-seated pathologies in cranial surgery, such as aneurysms, brain stem and skull base tumors, is challenging. The Surgeon-Controlled Robotics of ZEISS KINEVO 900 enables automated positioning to pre-defined anatomical landmarks based on pre-operative data planning –right when you need it.Save Move Recall7New dimensions. Freedom of choice. Working through oculars at extreme angles can sometimes be a pain in the neck. Literally. With no way out, you might have to contend with uncomfortable working positions causing fatigue. Now, relief and revolutionary dimensions in visualization arein sight.The Digital Hybrid Visualization with integrated 4K technology of ZEISS KINEVO 900 welcomes you to a world of heads-up ocular-free surgery, giving you freedom of movement. And freedom of choice to use an optical setup, depending on the application need.Fully integrated 4K camera technologyDuring lateral lumbar or thoracic spine and posterior fossa approaches,ZEISS KINEVO 900’s integrated 4K visualization can be essential. It providesyou with multimodal visualization capabilities – the flexibility to decouple fromthe classic optical approach and to work with outstanding 4K picture qualityand clarity. Even when magnifying tiny details.What’s more … your assistant surgeon, OR staff and residents also benefit from the 4K visual clarity of ZEISS KINEVO 900. They share the same high-resolution, digital image to follow the procedure with comparable fidelity. Delivering indispensable education and training.9Critical challenge. Vital solution.Your challenge: When working from an external perspectiveof a surgical microscope, your visualization of the anatomy is limited to a straight line of sight – missing critical information behind tissue or corners. Efficient and effortless access to this comprehensive information is essential for treatment.Our solution: QEVO from ZEISSThe unique, proprietary Micro-Inspection Tool from ZEISS complements intraoperative microsurgical visualization, enabling you to discover unexplored areas during the surgical intervention without additional footprint. You can look around corners and eliminate blind spots. And most importantly, you can gain greater insights – for better clinical decisions.To support your surgical workflow, ZEISS QEVO is engineered with an angled design – keeping your hands out of the lineof sight during insertion in the surgical field. And, it allowsfor an easy fit between the ZEISS KINEVO 900 and the situs, eliminating the need to reposition the head of the device. Greater insights, on demand.ZEISS QEVO enables you to inspect the perforator or examine the distal neck of the aneurysm to ensure the clip blades are fully extended.11Ease of use. Peace of mind.Surgical certainty is your imperative. Enabling you to achieve it is ours. That’s why, in the development of the Micro-Inspection Tool, we placed a high priority on its ease of use.ZEISS QEVO is truly integrated. You don’t have to plan for an additional device during surgery. Just plug it into your ZEISS KINEVO 900 for a seamless surgical workflow and to easily switch back and forth between views.ZEISS QEVO is fully autoclavable.So there’s no need forany additional draping. This is another attribute that makes ZEISS QEVO an indispensable tool – always available during surgery. On demand.ZEISS QEVO. Innovation in action.With its ability to look around corners, ZEISS QEVO enables identification of possible tumor remnants – avoiding unnecessary bone removal and retraction. During a Vestibular Schwannoma case, for instance, it can help identify the course of facial nerves. And, can support inspection of regions that are not directly visualized by a surgical microscope.131 142ZEISS BLUE 4002multi-center study1.ZEISS YELLOW 5602Real-time detection and visualization of malignant tissue during gliomasurgery using BLUE 400.Visualization of fluorescence-stained structures while performing left-temporal craniotomy for tumor resection using YELLOW 560. Obtained within the scopeof a clinical investigation.For a complete picture: The Diagram Functionoutlines assessment of fluorescence intensityvariation over time and fast access to the keyindicators for further analysis.For no compromises:AfterFor the fluorescence distribution: The IntensityMap enables you to conveniently identify relativefluorescence levels reached during the INFRARED800 observation period.For the speed of the flow: The Speed Mapindicates how fast the fluorescence intensityincreased during the observation period –indicating the speed of the blood flow.For the indicative time: The Delay Map (orSummary Map) provides quick information aboutthe time when the fluorescent signal appeared foreach image point in the map.Before15Setting new benchmarks. Shaping a new future. When we envisioned the all-new Robotic Visualization System,we conceived a design that can deliver so much more withoutlosing its familiarity. With ZEISS KINEVO 900, we continue tolive our vision of supporting you in becoming one with yourvisualization system – of delivering purposeful innovations.ones that matter the most for you.The Robotic Visualization System: The first of its kind.Surgeon-Controlled RoboticsDelivering precise positioning with a lotless effort – with motors in all axes.ZEISS QEVO – The Micro-Inspection ToolComplementing intraoperative microsurgicalvisualization to discover unexplored areasduring surgical intervention. Gain greaterinsight. On demand.16Digital Hybrid VisualizationProviding an opportunity for ocular-free surgery, with the freedom to use a traditional optical setup – depending on the application need.Integrated Intraoperative Fluorescence – The Power of Four.The redesigned intraoperative fluorescence technologies from ZEISS offer you the Power of Four – so you always have the tools you need.17Neurosurgery, in particular, is a technologically intensivesurgical discipline. This has pushed us toward the edge oftransformation: to develop leading digital technologiesenabling you to expand the boundaries of surgical care –to the next level.ZEISS KINEVO 900 offers full digital connectivity.Manage surgical data wherever you are: ZEISS Connect Appenables you to access your surgical data from your iOS device,and also delivers dedicated functionalities for efficient work-flows.Take teaching to new heights: ZEISS Observe App enablesyou to virtually broadcast your procedure in the OR. Yourstudents can follow the live surgery directly on mobile screensor immerse themselves in a rich VR Experience.Gain value with new digital services: ZEISS Smart Servicesenables faster support for you and your team with remoteconnectivity. Benefit from the increased system availabilitypowered by a secure connection to your ZEISS KINEVO 900.Digital connectivity. Transforming OR’s.ZEISS ConnectZEISS Observe18Connecting simplicity and innovation.ZEISS SMARTDRAPEYour visualization needs are paramount to us. And, soare the needs of your team. That’s why we gave a specialfocus to the OR preparation process in the developmentof ZEISS KINEVO 900.Being an integral part of the optical path, the SMARTDRAPEwith VisionGuard® from ZEISS is designed together withZEISS KINEVO 900 so you and your team can have thebenefits of a vivid view, and effective patient protection.At the same time – the new innovations make the drapingprocess simply simple!• Innovative folding: to eliminate guesswork and complexity.• Intuitive attachment: for an effortless and simple self-locking mechanism.• Integrated RFID chip: for easy activation of AutoDrape®.Designed for ZEISS KINEVO 900.Support whenever you need it.ZEISS OPTIMEIf you rely on high system availability, consider our ZEISSOPTIME service agreements, which are designed to ensurethe readiness of our medical equipment when you need it.ZEISS OPTIME service agreements for ZEISS KINEVO 900now come with connectivity for ZEISS Smart Services.19Tr a n s p or t d i r e c t io n 850 m mma x.c a .1760m m c a . 530 - 1635 m m-25° / +135°A x i s 4±45°A x i s 5-28° / +20°A x i s 3-A x i s M o n i t o r R o t a t e : ±125°T i l t :-20° / +5° (±3°) 360° c o n t i n u os A x i s1 25° / +225°A x i s 6T o le r a n c M in .M i n .M in .Technical DataKINEVO ® 900 from ZEISS Technical DataRated Voltage 100 V – 240 VCurrent Consumption Max. 1.350 VARated Frequency 50 Hz – 60 HzElectrical Standard Complying with IEC 60601-1:2005/A1:2012Protection class I, degree of protection IP20Class 2 laser product as perIEC 60825-1:2007, IEC 60825-1:2014Weight Weight max. 395 kgWeight of system incl. transport container: 20Cable length: 5 mQEVO ® from ZEISS and QEVO ECUTechnical Data Direction of View 45° upwards Shaft Diameter 3.6 mm Shaft Length 120.0 ± 1.0 mm Total Diameter 13.0 mmField of View 100° +5°/-10° (ISO 8600-3:2019-08)Illumination20 – 35 lumen LED Weight (without cable)250 g Sterilization AutoclavableImage Resolution 1920 x 1080 pixel full HD Length of Cable5000 mmOperation Temperature +10 to +40 °C (500/1000 s intermittent use)QEVO ECU Dimensions Length = 265.0 ± 1 mm, height = 59.3 ± 1 mm and depth = 212.2 ± 1 mm Weight2.2 kgOperating Voltage 24V (+/- 10%) ADC Video OutputDVI-D full HD21Technical DataOptions VideoStereo video camera 3D HD, fully integrated, 2 x 3-chip HD, 1080p incl. 2nd HD 3D monitor 4K video camera, fully integrated 3-chip 4K, 2160p Stereo video camera 4K 3D, fully integrated, 2 x 3-chip 4K, 2160p, incl. 2nd HD 3D monitor Integrated HD video recording, withSmartRecording, low-Resolution recording, editing and streaming 2nd system monitor HD 2DAttachment for consumer (SLR) photo camera External 55" 4K 3D video monitor, with mobile cartIntraoperative FluorescenceBLUE 400INFRARED 800INFRARED 800 Compact INFRARED 800 with FLOW 800YELLOW 560Connectivity / Data Manage-mentDICOM module for image and video data transfer from / to PACS. Patient management by modality worklist management.Shared Network Data storage WLAN option, with WiFi Hotspot Navigation Interface Standard Navigation Interface ExtendedAccessoriesZEISS QEVO and QEVO ECU12.5x magnetic wide field eyepieces with integrated eyecups Stereo co-observation tubeFoldable Tube f170 / f260, including the PROMAG function for additional 50 % magnification and integrated rotate functionTiltable binocular tube, swivel range 180°, focal length f = 170 mm14-function, wired foot control panel 14-function, wireless foot control panel 2-function foot switch Mouth switch3-step magnification changerStandard Configuration Apochromatic OpticsMotorized focus; Varioskop ® with working distance 200 – 625 mmMotorized zoom; zoom ratio 1:6, magnification factor y = 0.4x – 2.4x10x magnetic wide field eyepieces with integrated eyecupsAutoFokus with 2 visible laser dots, automatic mode with magnetic brakesIllumination2 x 300 W Xenon, with automatic lamp exchange Automatic Iris Control for adjusting the illumination to the field of view Individual light threshold settingFocus Light Link: working distance controlled light intensityManual adjustment of diameter of field of illuminationAdditional illumination beam to brighten up shadows, motorizedSystem OperationMultifunctional programmable handgrips Magnetic clutches for all system axes Central user interface with full-screen video XY robotic movement in 6 axes (variable speed)Active dampingManual and motorized PointLock function with variable speedPositionMemory (with variable speed)Motorized XY lateral movement with variable speedMultiVision System (HD), with shutter controlSystem SetupAutoBalanceAutoDrape – air evacuation system 1Park Position Drape PositionVideoIntegrated 3-chip Full HD video camera, 1080p 24" HD video touchscreen on extendable arm, 16:9 aspect ratioIntegrated still image capturing both on HDD and USB-mediaConnectivity / Data Manage-ment Video-in for external HD video sources Remote diagnosis via internet / VPN Sterile DrapeZEISS SMARTDRAPE1Available with ZEISS SMARTDRAPE only.22Your needs. Our packages.Select a ZEISS KINEVO 900 built to fit your typical clinical use-cases. ZEISS KINEVO 900 comes with pre-defined packages giving you a head start in planning the most suitable configuration for your specific needs.Interested in digital visualization? Check out the digital package. That’s our commitment to cover you for tomorrow while keeping your present needs into focus.always included always included as INFRARED 800 only optional23e n -O U S _30_010_0097I I P r i n t e d i n G e r m a n y . C Z -V I I /2021T h e c o n t e n t s of t h e b r o c h u r e m a y d i f f e r f r o m t h e c u r r e n t s t a t u s o f a p p r o v a l o f t h e p r o d u c t o r s e r v i c e o f f e r i ng i n y o u r c o u n t r y . P l e a s e c o n t a c t o u r r e g i o n a l r e p r e s e n t a t i v e s f o r m o r e i n f o r m a t i o n . S u b j e c t t o ch a n g e si n d e s i g n a n d s c o p e o f d e l i v e r y a n d d u e t o o n g o i n g t e c h n i c a l d e v e l o p m e n t . R o b o t i c V i s u a l i z a t i o n S y s t e m , K I N E V O , Q E V O , F L O W , A u t o D r a p e , V a r i o s k o p , S M A R T D R A P E a n d V i s i o n G u a r d a r e e i t h e r t r a d e m a r k s o r r e g i s t e r e d t r a d e m a r k s o f C a r l Z e i s s M e d i t e c A G o r o t h e r c o m p a n i e s o f t h e Z E I S S G r o u p i n G e r m a n y a n d /o r o t h e r c o u n t r i e s .© C a r l Z e i s s M e d i t e c A G , 2021. A l l r i g h t s r e s e r v e d .Carl Zeiss Meditec AG Goeschwitzer Strasse 51–5207745 Jena Germany/med /kinevoKINEVO 900QEVO ECU BLUE 400YELLOW 5600297QEVOINFRARED 800 with FLOW 800 Option SMARTDRAPEView onto cerebellum and lower cranial nerves. Image courtesy of Dr. Robert F. Spetzler, Barrow Neurological Institute, Phoenix, Arizona, USA. (Cover page) Front temporal area for STA-MCA bypass procedure. Image courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA (Cover page)Aneurysm clipping using ICG and overlay . Image courtesy of Prof. Dr. Andreas Raabe, Inselspital, University Hospital of Bern, Switzerland (Page 2 and 3)View onto optic nerve and internal carotid artery. Image courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 4)Image-guided surgery. Image courtesy of BrainLab AG (Page 6 and 7)View onto spinal cord dura. Image courtesy of Dr. Robert F. Spetzler, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 8 and 9)Small view of the cerebellum through the Retrosigmoid Approach. Image courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 10)Left mini-pterional approach for clipping an aneurysm. Image courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA (page 11)View onto corpus callosum and septum pellucidum. Image courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 12)Transnasal transspenoidal for re-exploration and excision of recurrent pituitary Macroadenoma with possible abdominal fat. Image courtesy of Dr. William White, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 13)Hemmorrhage from right temporal AVM. Image courtesy of Dr. Gary K. Steinberg, MD PhD, Stanford University (Page 14)Right temporal Craniotomy for AVM. Image courtesy of Dr. Robert F. Spetzler, Barrow Neurological Institute, Phoenix, Arizona, USA (Page 15)Glioma surgery using BLUE 400. Image courtesy of Prof. Dr. Walter Stummer, University Clinic, Münster, Germany (Page 15)Left-temporal craniotomy for tumor resection with YELLOW 560. Image Courtesy of Dr. Peter Nakaji, Barrow Neurological Institute, Phoenix, Arizona, USA. (Page 15)。


附录II技术文档制造商拟定的技术文档及其摘要(如适用)应以清晰、有条理、易于搜索和明确的方式呈现,尤其应包括本附录中列出的要素。
1、产品描述和特征,包括变体和附件/配件1.1、产品描述和规格a)产品或贸易名字和产品基本描述包括预期用途和预期使用人群b)附录VIPartC提到的制造商分配给器械的UDI-DL基于UDI系统,或产品代码,序列号或其他可参考的标识就会被立马识别c)预期用途包括以下信息:I)检测或测量物质II)功能,例如:筛查、监测、诊断或辅助诊断、预测、伴随诊断III)旨在检测、定义或区分特定疾病、情况和风险因素IV)自动或非自动V)定性、半定量或定量VI)样本类型VII)预期检测人群VIII)预期使用者IX)另外,伴随诊断需要有相关目标人群和相关药品d)检测方法或仪器操作原则的描述e)产品作为医疗器械的理由f)风险等级和分类规则的应用遵循附录VIIIg)组分的描述,和相关组分里活性成分:抗体、抗原、核酸引物(如适用)如适用:h)样本采集、和器械一起提供的运输材料,或推荐使用规格的描述i)对于自动检测的仪器:描述适用的检测性能或专用检测性能j)对于自动检测试剂:描述适用的仪器性能或专用的仪器性能k)描述与器械一起使用的软件引申:TUV l) 拟投放市场的器械的各种配置/变体的说明或完整列表m )描述预期与器械联合使用的配件、其他器械和非器械引申:TUV 解读,单独提供的配件需要单独的标签、说明书、包装和证书1.2、a) 己上市同类产品或制造商已制造产品的概览b) 已在国际市场上销售的类似产品的概览2、制造商提供的信息a) 器械上的标签或包装(例如单一包装、销售包装、运输包装)的语言应被预想销往的成员国接受b )使用说明书语言应该被预想销往的成员国接受3、设计和制造消息3.1、设计信息用于理解器械设计阶段的信息应包括:a )描述与器械一起提供或推荐一起使用的关键组分如抗体、抗原、酶和核酸引物b )对于仪器:描述主要子系统、分析技术例如操作原则、控制原理、专用电脑硬件或软件c )对于仪器和软件:描述整个系统d )对于软件:描述数据解读技术及算法e )对于预期用于自我测试或近患者测试的器械:描述自我测试或近患者测试的适用性设计方面3.2、制造信息a )生产制造过程信息,例如生产过程、装配、成品测试、成品的包装信息。

产品系列手册PTFE 内衬对比手册从柔软、灵活到刚硬,Zeus 多样化的高性能薄壁导管内衬产品组合可帮助设备工程师将今天的不可能变成明天的现实。
|*******************PTFE 内衬对比手册导管内衬?让我们为您服务。
内衬选择仍然是导管设计成功的基石。
一般而言,导管内衬最需要的特征是薄壁和低摩擦系数(高润滑性)。
尽管如此,对于每个导管项目,医疗器械制造商都必须将应用要求与适用内衬的尺寸和机械性能相匹配。
润滑性 -薄壁 -可使用多种材料来制造导管内衬,然而,尽管有许多可供选择的材料,但由于其出色的润滑性,PTFE 在很大程 度上仍然是大多数导管设计的黄金标准。
PTFE 在所有聚合物中具有最低的摩擦系数 – 在考虑导管的内径 (ID ) 时, 这个特征非常重要。
导管内径润滑性的增加可降低腔内装置 (如支架、球囊等) 在通过导管工作通道时的展 开力,从而增加手术成功的可能性。
PTFE 除具有较低的摩擦系数外,该材料还能够加工成具有极薄壁和严格公差的管道,这是导管设计中的另一个关键优势。
具有极薄壁的导管内衬让设计人员能够实现导管工作通道的最大化,同时可将器械的整体尺寸保持在最低限度。
薄壁也是影响导管强度和灵活性的一个因素 -- 然而,除导管内衬的尺寸之外,内衬的实际生产方式对其整体性能也起到重要作用。
强度和柔韧性 -目前,存在多种方法可用于生产薄壁 PTFE 内衬:铸膜、自由挤出和挤出覆线管 (OTW)。
每种生产方法所生成的导管内衬具有略微不同的特征和设计考虑因素。
例如,自由挤出可使 PTFE 基体中的晶粒松开,形成轴向或挤出方向的原纤维。
这些原纤维有助于形成自由挤出内衬中常见的强度和刚度。
铸膜不会让单个的 PTFE 分子链产生分子取向。
与同等尺寸的自由挤出内衬管相比,具有这一特点的内衬管虽然较弱,但更加柔韧。
最后,挤出覆线管会导致一些分子定向,因此这些内衬表现出介于自由挤出和铸膜内衬之间的特征。

F1173KReverTra Ace® qPCR RT Master MixFSQ-201 200 reactionsStore at -20°C Contents[1] Introduction[2] Components[3] Protocol1. RNA template for reverse transcription2. Reverse transcription[4] Application data[5] Related protocol1. DNase I treatment of total RNA[6] Troubleshooting[7] Related productsC AUTIONAll reagents in this kit are intended for research purposes. Do not use for diagnostic or clinical purposes. Please observe general laboratory safety precautions while using this kit.-ReverTra Ace® is a registered trademark of Toyobo Co., Ltd., Japan.JAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio 1JAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio********************1[ 1 ] Introduction [ 2 ] Components DescriptionReverTra Ace® qPCR RT Master Mix is an efficient and convenient reagent to synthesize high quality cDNAs for real-time PCR. The master mix reagent (5x) contains the highly efficient reverse transcriptase “ReverTra Ace®”, primers and buffer optimized for highly efficient synthesis of short-chain cDNAs suitable for real-time PCR. The protocol is simple, and the reaction can be completed in 15 min.ReverTra Ace® is a mutant M-MLV reverse transcriptase that shows excellent efficiency. Features-5x Master Mix reagent contains all components for reverse transcription.The Master Mix reagent will not freeze at -20°C.-No reverse transcription control experiments (no RT-Control) can be performed.-The master mix reagent contains random and oligo dT primers optimized for efficient reverse transcription.-The reaction can be completed in 15 min. The protocol does not contain an additional RNase H treatment step to remove residual RNA after reverse transcription (Patent Pending).-Since the RT buffer is optimized for real-time PCR, the addition of 20% (v/v) of the synthesized cDNA solution to the PCR solution does not inhibit the PCR reaction.Therefore, this kit is suitable for the detection of low abundance mRNAs.The kit includes the following reagents, which can be used for 200 (FSQ-201) and 40 (FSQ-201S) 10 µl reactions. All reagents should be stored at -20°C. For extended storage, -30°C is recommended.FSQ-201FSQ-201S (SAMPLE) 5x RT Master Mix 400 μl80 μl5x RT Maser Mix no RT-Control 40 μl8 μlNuclease-free water 1000 μl x 2400 μl5× RT Maser MixThis reagent is a 5x master mix that contains highly efficient reverse transcriptase “ReverTra Ace®”, RNase inhibitor, oligo dT primer, random primer, MgCl2 and dNTPs .NotesBe aware that “5x RT Master Mix” and “5x RT Master Mix II” in ReverTra Ace® qPCR RT Master Mix with gDNA remover (Code No. FSQ-301) are not compatible.5× RT Maser Mix no-RT ControlThe composition of “5x RT Master Mix no-RT Control” is identical to that of “5x RT Master Mix” except that the reverse transcriptase (RT) is omitted. This master mix can be used in control experiments due to the absence of reverse transcriptase.Nuclease-free waterThis nuclease-free water has been prepared without DEPC treatment.JAPAN CHINA TOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140 www.toyobo.co.jp/e/bio********************2[ 3 ] Protocol1. Template RNA for reverse transcriptionThe following RNAs are appropriate for highly efficient reverse transcription.(1)Total RNATotal RNA usually contains 1-2% mRNA. Total RNA can be used directly as template with this kit. RNA prepared using acid guanidium-phenol-chloroform (AGPC) or the spin-column method contains genomic DNA, so total RNA should be treated with DNase I before transcription.(2)Poly(A)+ RNA (mRNA)Poly(A)+ RNA is useful to detect low abundance mRNAs. However, poly(A)+ RNA should be treated carefully because it is more sensitive to RNase than total RNA.2. Reverse transcription(1) Denaturation of RNA [optional]Incubate the RNA solution at 65°C for 5 min, and then keep on ice.Notes-This step increases the efficiency of reverse transcription of RNA templates that form secondary structures.-This step should be performed before adding 5x RT Master Mix.(2) Preparation of the reaction solutionPrepare the following reagents on ice.Notes-The master mix reagent contains oligo dT and random primers. Do not use with specific primers.-For control experiments, “5x RT Master Mix no RT-Control” should be used instead of 5x RT Master Mix. A control experiment without reverse transcription is useful to prove whether amplicons originate from cDNA and/or genomic DNA.-The reaction volume can be increased according to need.-Master mix reagents should be spun-down prior to use due to high viscosity.5x RT Master Mix2 μl RNA template 1 pg – 1 μgNuclease-free Water X μl Total V olume10 μlJAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio********************3-This kit contains nuclease-free water for 200 reverse transcription reactions. The kit does not contain sufficient nuclease-free water for the dilution of RNA samples.Nuclease-free water prepared without DEPC-treatment is recommended for the dilution of RNA samples.(3)Incubate at 37°C for 15 min(4)Incubate at 50°C for 5 min [optional](5)Heat to 98°C for 5 min(6)Store the reacted solution* at 4°C or – 20°C*This solution can be used directly or after dilution for real-time PCR.Notes-The reaction time at 37°C can be prolonged up to 1 hr.-ReverTra Ace® excels at high reaction temperatures (up to 50°C). This step may increase the efficiency of the reverse transcription.-Up to 20% of the synthesized cDNA solution can be added to the PCR reaction solution.JAPAN CHINA TOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140 www.toyobo.co.jp/e/bio********************4[ 4 ] Application data<Materials and Methods>cDNA synthesis Reagent: ReverTra Ace ® qPCR RT Master Mix (Code No.FSQ-201) Template: HeLa total RNA 2 pg-2 μg /20 μl reactionReal-time PCRReagent: THUNDERBIRD ® SYBR ® qPCR Mix (Code No.QPS-201) Template: cDNA 2 μl/20 μl reaction (cDNA solution: 10%) Targets: Typical house-keeping genes Real-time cycler: Applied Biosystems 7900HT<Results>Template RNA (pg)Log (RNA amount)Ct of qPCRATP5F TFRC RPLP1 RPLP2 RPS182 0.301 33.76 31.16 32.89 32.5420 1.301 31.43 30.73 27.70 30.05 28.65 200 2.301 28.64 27.29 24.44 26.72 25.22 2,000 3.301 25.41 23.79 21.12 23.31 21.98 20,000 4.301 21.86 20.43 17.69 19.88 18.42 200,000 5.301 18.65 17.09 14.14 16.59 15.10 1,000,000 6.000 16.03 15.03 11.63 14.37 13.09 2,000,000 6.301 15.42 14.28 11.11 13.53 12.28/20μl Slope -3.280 -3.303 -3.384 -3.284 -3.368R20.999 0.999 1.000 1.000 0.999 Eff.101.8%100.8%97.5% 101.6% 98.1%High linearity and no crossing over of the standard curves of five housekeeping genes suggest that the reagent shows high performance in a broad concentration range.JAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio********************5[ 5 ] Related Protocol1. DNase I treatment of total RNATotal RNA prepared by general methods contains genomic DNA. Genomic DNA can beeliminated by the following method.(1)Mix the following reagents.Nuclease-free water X μlTotal RNA (<10 μg)Yμl10 x DNase I Buffer[e.g. 100 mM Tris-Cl, 20 mM MgCl2(pH 7.5)]1 μlRNase-free DNase I (10 U/μl)0.5μlTotal volume 10 μl(2)Incubate on ice for 10-30 min.(3)Purify the treated RNA according to the following step.DNase I-treated RNAAdd nuclease-free water (adjust volume to 100 μl)Add 100 μl TE-saturated phenolVortexKeep on ice for 5 minCentrifuge at 12,000 rpm for 5 minSupernatantAdd 100 μl chloroform: isoamyl alcohol (24:1), V ortexCentrifuge at 12,000 rpm for 5 min at 4 °CSupernatantAdd 100 μl 5 M ammonium acetate + 200 μl isopropanol+ [5 μl 2 mg/ml glycogen* (for coprecipitation) : optional]VortexIncubate at - 20 °C for 30 minCentrifuge at 12,000 rpm for 10-15 min at 4 °CDiscard supernatantPrecipitateAdd 1 ml 70% ethanolCentrifuge at 12,000 rpm for 5 minDiscard supernatantPrecipitateDissolve in appropriate volume of nuclease-free waterRNA solution*Molecular biology gradeJAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio********************6[ 6 ]TroubleshootingSymptom Cause SolutionLow signal afterreal-time PCRLow purity of RNA Repurify the RNA sample.Degradation of RNA Prepare fresh RNA sample. Diluted RNA templates havea tendency to degrade and to adsorb on the vessel walls.RNA template for the reaction should be prepared from ahighly concentrated stock prior to use.Excess or small amount of RNA The recommended RNA concentration range for reverse transcription is from 1 pg to 1 μg in a 10 μl reaction. However, the optimal concentration of RNA template should be determined for each case.Secondary structure of RNA template The efficiency of reverse transcription of RNAs that form secondary structures tends to be low. Incubation at 65°C for 5 min and quenching prior to the reaction is usually effective on such templates. Also, the additional step of 50°C for 5 min after the reaction at 37°C for 15 min might be effective for such difficult templates.Inappropriate temperature conditions Perform the reaction according to this instruction manual.Excess amount of cDNAsolution compared to thetotal PCR reaction volumeReduce the cDNA solution to less than 10%.Amplification in no-RT control reaction Contamination of genomicDNA in RNA templateRedesign the primers to prevent amplification fromgenomic DNA. Or treat the template RNA with DNase Iprior to reverse transcription.Primer dimer formation Optimize the PCR conditions or redesign the primers.HPLC-grade primers sometimes improve PCRspecificity.JAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio********************7[ 7 ] Related productsProduct name Package Code No.High efficient revers transcriptaaseReverTra Ace®10,000 U TRT-101RNase inhibitor (Recombinant type) 2,500USIN-201 Real-time PCR master mix for probe assayTHUNDERBIRD® Probe qPCR Mix 1.67 mL x 3 QPS-101Real-time PCR master mix for SYBR® Green assayTHUNDERBIRD® SYBR® qPCR Mix 1.67 mL x 3 QPS-201High efficient cDNA synthesis kit for Real-time PCRReverTra Ace® qPCR RT Kit200 reactions FSQ-101High efficient cDNA synthesis master mix Real-time PCR with gDNA removerReverTra Ace® qPCR RT Master Mixwith gDNA remover200 reactions FSQ-301。


产 品 规 格 书 Product Specification文件编号 Document number WE-WI-RD-962 版本版次Version editionA/0产品名称 Product name 3535内封ICN302B7产品规格Product specificationWE-3535AY0203Z-001文件编制 Documentation 马明海 批准发行Approved issue马小其客户服务 Custormer Service 联系电话 Contact number客户名称 Customer name 样品编号 Sample number产品验证 Product verification批准承认 Recognition approval注: 1.此规格书以中英文方式书写,若有冲突以中文版文本为准。
/This specification is written in both Chinese and English. In case of conflict, the Chinese version shall prevail.2.此规格书的最终解释权归由本公司。
/The final interpretation of this specification shall be vested in the company.目 录Catalog0.0、封面/co ver ..........................................................................第1页page 10.1、目录/Catalog ......................................................................... 第2页page 21、产品概述/Product Overview ..............................................................第3页page 32、功能特点/Functional characteristics.....................................................第3页page 33、应用领域/Application area ..............................................................第3页page 34、外观描述 /Appearance description ......................................................第4页page 45、封装尺寸/Size .........................................................................第4页page 46、脚位图/Foot map .........................................................................第5页page 5第5页page 5 7、最大额定值/MaximumRating ............................................................第6页page 6 8、推荐工作范围/Recommended scope ofwork .................................................9、电气参数/Electrical parameters..........................................................第6页page 610、开关特性/Switching第7页page 7 characteristics.....................................................11、内置LED参数/Built-in LED parameters..................................................第7页page 712、功能说明/Description of functions......................................................第7页page 713、恒流曲线/Constant-current第10页page 10 curve........................................................14、应用线路图/Application Route Diagram..................................................第10页page 1015、使用注意事项/Precautions ............................................................第11页page 111、产品概述/Product Overview:N302B7-3535RGB是一款集成高质量单线级联恒流驱动IC N302B7和高质量RGB LED芯片的外控恒流3535集成灯珠。

风采金域:16年医学实验室国际质量管理体系建设之路文/庞颖2018世界认可日前夕,金域医学广州实验室先后迎来了ISO 15189扩项+换证评审,以及C A P(美国病理学家协会)复评审。
从2002年率先导入I S O/I E C 17025体系,到2009年成功拥有国内首家C A P与I S O 15189《医学实验室和能力认可准则》双认可实验室,再到如今的国内外28张认证认可证书,金域医学的医学实验室国际质量管理体系建设之路已经走过了16年。
不仅为第三方医学检验行业树立了质量的“金标准”,带领行业良性发展,更为助力国家建设一系列新进医学实验室的技术质量标准,推动国家医学检测行业进步,为健康中国建设贡献力量。
在医学实验室率先践行17025体系90年代,当国内第三方医学检验仍处空白时,金域医学的核心创业团队就开始探索医学检验外包服务在中国的运营模式。
当时到处充斥着质疑的声音,认为连医院都没法做的检验凭什么相信一个第三方的医学实验室?回想起创业初期的艰难,金域医学副总裁胡朝晖认为,做医学检验,质量是核心,直接影响到检测、诊断结果是否准确可信。
最终,金域医学选择了用质量说话。
然而,当时国内只有实验室间质量评价,还没有专门的医学实验室质量管理体系标准。
金域医学经过深入调查,决定双管齐下:一方面,率先实践国际通用的检测与校准实验室质量管理体系标准(ISO/IEC 17025);另一方面,积极参加国家的实验室间质量评价。
2002年,金域通过了ISO/IEC 17025,这是一个对所有检测和校准实验室通用质量管理体系标准。
金域医学是率先将实验室质量管理体系引入的医学实验室,并获得ISO/IEC 17025国际认可,成为了中国临床医学实验室质量管理体系建设的第一批探索者。
为第三方医检行业发展树立质量标准金域医学对质量的追求并不满足于此,为了进一步保障临床检验报告的质量,金域医学对自己提出了三方面更高要求。
首先,追求卓越,拓宽质量范畴。

1IndIcatIons for Use/Intended UseThe MyoSure ® Instrument Tray is intended to enclose, protect, and organize the MyoSure ® Rod Lens Hysteroscope, MyoSure ® Removable Outflow Channel, and associated components, during sterilization and storage. The MyoSure ® Instrument Tray must be used in conjunction with a sterilization wrap that is cleared by FDA for the indicated sterilization cycle, and may be stored for up to 30 days in accordance with the wrap manufacturer’s instructions.The MyoSure ® Instrument Tray has been validated for the following sterilization cycles:Prevacuum Steam Gravity Steam STERRAD ® 100S™132°C (270°F) 132 °C (270 °F)59% H2O24 minutes expo-sure15 minutes exposure Normal Cycle Setting Normal Cycle Setting 30 minutes dry time 30 minutes dry time Contents - 1MyoSure ® Rod Lens Hysteroscope, 1 Removable Outflow Channel, 2 Seal Caps, 2 Light GuidesContents - 1MyoSure ® Rod Lens Hysteroscope, 1 Removable Outflow Channel, 2 Seal Caps, 2 Light GuidesContents - 1MyoSure ® Rod Lens Hysteroscope, 1 Removable Outflow Channel, 2 Seal Caps, 2 Light GuidescontraIndIcatIonsStacking of trays and overloading of the units will adversely affect sterilization and drying effectiveness. DO NOT STACK trays in the sterilization chamber.care and cleanIng recommendatIons• Pre-Rinse soiled trays in tap water for 2 minutes• Both physical and chemical (enzymatic detergent) processes may be necessary to clean soiled items. Follow enzymatic detergent manufacturer’s instructions• Chemical (enzymatic detergent) cleaners alone may not remove all soil and debris; therefore, a careful manual cleaning of each item with a soft sponge or cloth is essential.• For difficult access areas, a clean, soft-bristled brush is recommended.• Repeat cleaning steps if visible contamination of tray components is observed.•Once the items have been cleaned, they should be thoroughly rinsed with clean water to remove any detergent or chemical residue before sterilization.• Hologic recommends the use of a mild enzymatic detergent with a near neutral pH.• Do not use solvents, abrasive cleaners, metal brushes, or abrasive pads.• Trays may be placed in mechanical cleaning equipmentCAUTION: Always inspect tray components for cleanliness and confirm that there is no visual contamination prior to use.CAUTION: Trays are reusable but may eventually be damaged. Always inspect tray components for cracking, chipping, or other signs of damage before use. Make sure all latches and handles are secure and in working order. Damaged trays should be removed from service. Minor Surface cosmetic changes may occur with long-term use or after Sterrad ® processing.CAUTION: Only use accessory components that have been designed and tested for use in MyoSure ® Instrument Trays.sterIlIzatIon recommendatIonsDo not overload trays. Place and arrange tray contents in accordance withFigure 1 to facilitate sterilant contact with all objects in the tray.Figure 1Process the tray according to the sterilization wrap manufacturer’s instructions prior to sterilization to maintain sterility of internalcomponents/items and for proper aseptic presentation to the surgical field. Product may be maintianed for up to 30 days after sterilization in accordance withthe wrap manufacturer’s intructions.steam sterIlIzatIonPerformance of the MyoSure ® Instrument Tray has been verified in the following steam autoclave cycles for sterilization of the MyoSure ® Rod Lens Hysteroscope and accessory components.Instrument tray Instructions for Use2MAN-02848-001 Rev. 002 02-2013whenever handling a load after a cycle cancellation, or if any moisture is noted on a load following a completed cycle.servIce – accessorIesThe following are replacement parts for the MyoSure ®Instrument Tray:WarrantY, servIce, and rePaIr WarrantY InformatIonTHE MYOSURE ® INSTRUMENT TRAY AND ACCESSORIES ARE PROVIDED “AS IS” WITH NO WARRANTY, EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. CUSTOMER IS RESPONSIBLE FOR ANY REPAIR OR REPLACEMENT OF THE MYOSURE ® INSTRUMENT TRAY.tecHnIcal sUPPort and ProdUct retUrn InformatIonContact Hologic Technical Support if the MyoSure ® Instrument Tray fails to operate as intended.Hologic Technical Support United States:Phone: 1.800.442.9892 (toll-free) or 1.508.263.2900 Fax: 1.508.229.2795for fUrtHer InformatIonIf further information on this product is needed, please contact Hologic Customer Service at 800-442-9892 in the U.S., or your authorized representative.In USA contact:Hologic, Inc.250 Campus DriveMarlborough, MA 01752Toll Free Phone: 1-800-442-9892Toll Free Fax: 1-800-409-7591Hologic, MyoSure, and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and other countries. All other trademarks, registered trademarks, and product names are the property of their respective owners.The MyoSure instrument tray is manufactured for and distributed by :250 Campus DriveMarlborough, MA 01752 USA©2013 Hologic, Inc. All Rights Reserved.tray, instrument configuration, total contents of the sterilizer, steam quality, equipment maintenance, and others.CAUTION: Do not load trays into sterilizer on sides or upside down with lid side on the shelf or cart. Load trays on cart or shelf, so that the lid is always facing upward. This will allow for proper drying. The MyoSure ® Instrument Tray is designed to drain in this position.CAUTION: After the autoclave door is opened, all trays must be allowed to cool thoroughly. Place trays on a rack or shelf with linen cover until cooling is complete. The potential for condensation may increase if the tray is not allowed to cool properly.If condensation is observed, verify that the steam, which is being used for sterilization processing, has a quality of more than 97%. Also confirm that the sterilizers have been inspected for routine maintenance in accordance with manufacturer recommendations.sterrad ® sterIlIzatIonThe MyoSure ® Instrument Tray has been validated for use with the STERRAD ®100S™ System.Refer to the STERRAD ® 100S™ User’s Guide for additional warnings, precautions, and more information about the STERRAD ® 100S™ sterilization process.WARNING: Hydrogen peroxide is corrosive and concentrated hydrogen peroxide is toxic. Wear chemical resistant latex, PVC (vinyl), or nitrile gloves。

协议书合同甲方:金域医学乙方:___________鉴于甲方是一家专业从事医学研究和服务的机构,乙方希望利用甲方的专业知识和设施进行相关的研究和服务,双方本着平等互利的原则,经友好协商,达成如下协议:一、合作内容1.甲方为乙方提供医学研究服务,包括但不限于实验室检测、数据分析、科研咨询等。
2.甲方为乙方提供医学培训服务,包括但不限于专业知识培训、技能培训等。
二、合作期限本协议自双方签字盖章之日起生效,有效期为____年,除非双方另有约定。
三、费用及支付1.乙方应支付甲方提供的服务费用,具体金额由双方另行协商确定。
2.乙方应在服务完成后____日内支付服务费用。
四、保密条款1.双方在合作过程中所获悉的对方商业秘密、技术秘密等保密信息,应予以严格保密。
a)该信息已为公众所知;b)该信息在披露前已为接收方所拥有;c)该信息由第三方合法提供,且未附加保密义务。
五、违约责任1.双方应严格按照本协议的约定履行各自的义务,如一方违约,应承担违约责任。
2.违约方应赔偿对方因此造成的损失,包括但不限于直接损失、可得利益损失、律师费等。
六、争议解决如双方在履行本协议过程中发生争议,应通过友好协商解决;协商不成的,任何一方均有权向甲方所在地的人民法院提起诉讼。
七、其他1.本协议一式两份,甲乙双方各执一份。
2.本协议未尽事宜,双方可另行签订补充协议,补充协议与本协议具有同等法律效力。
甲方:金域医学乙方:___________签订日期:__________协议书合同甲方:金域医学乙方:___________金域医学合同模板概述:本协议旨在明确甲乙双方在医学研究和服务方面的合作事宜,包括服务内容、费用支付、保密义务等,以保障双方权益,促进合作顺利进行。
一、合作内容1.甲方为乙方提供医学研究服务,包括但不限于实验室检测、数据分析、科研咨询等。
2.甲方为乙方提供医学培训服务,包括但不限于专业知识培训、技能培训等。
二、合作期限本协议自双方签字盖章之日起生效,有效期为____年,除非双方另有约定。


(KM知识管理)KM培训业务操作手册实验室理化遗传广州金域集团业务核心系统业务操作手册模块:实验室主编:编写委员会(排名不分先后,以姓名字母为序):编写组:©2015广州金域集团版权所有。
保留所有权利。
没有广州金域集团的特别许可,任何人不能以任何形式或为任何目的复制或传播本文档的任何部分。
Microsoft、Windows、Outlook和PowerPoint是MicrosoftCorporation的注册商标。
IBM、DB2、DB2UniversalDatabase、OS/2、ParallelSysplex、MVS/ESA、AIX、S/390、AS/400、OS/390、OS/400、iSeries、pSeries、xSeries、zSeries、z/OS、AFP、IntelligentMiner、WebSphere、Netfinity、Tivoli和Informix是IBMCorporation在美国和/或其他国家的商标或注册商标。
Oracle是OracleCorporation的注册商标。
UNIX、X/Open、OSF/1和Motif是OpenGroup的注册商标。
Citrix、ICA、ProgramNeighborhood、MetaFrame、WinFrame、VideoFrame、和MultiWin是CitrixSystems公司的商标或注册商标。
HTML、XML、XHTML和W3C是W3C®麻省理工学院WorldWideWeb协会的商标或注册商标。
Java是SunMicrosystems公司的注册商标。
JavaScript是SunMicrosystems公司的注册商标,由其技术开发和实施商Netscape许可使用。
MaxDB是MySQLAB,Sweden.的商标。
本文档提到的相关产品和服务以及它们各自的徽标是其在本国和世界其它一些国家的商标或注册商标。
本文档提到的所有相关产品和服务名称是它们各自公司的商标。


首页格式要求:说明书首页规范格式应该无页眉和页脚。
首页内容应该包含说明书的名称、适用设备型号范围、适用的软件版本、版本信息、公司LOGO、发行日期等其他信息。
产品名称和产品类型必须分两行单独列出,建议使用相同大小的字体。
目录目录部分的页眉、页脚的页码应该使用I、U、川类型的符号页码。
其中页眉应该包含公司LOGO,公司名称,说明书的名称,如下:自探圳市董康生物科技有限公阿口诲曲AE全自动酶舷愿说明书z 页脚部分,如下:版報所有◎ 2010-20K f剁II市爰康生物科技有限公司-保留所耳权利-心目录格式目录仅显示前3级标题。
一级标题:宋体+Arial,小四,加粗,左对齐,段前6磅,段后6磅,单倍行距。
二级标题:宋体+Arial,小四,左对齐,左缩进0.37厘米,段前0行,段后0行,单倍行距。
三级标题:宋体+Arial,五号,左对齐,左缩进0.74厘米,段前0行,段后0行,单倍行距。
标题格式一级标题:黑体+Arial,加粗,三号,两端对齐,大纲级别1级,悬挂缩进0.76厘米,段前0行,段后0行,行距多倍行距,设置值2.41,非正规编号格式“第*章”编号位置左对齐,对齐位置0厘米,制表位位置0.76厘米,缩进位置0.76厘米。
二级标题:宋体+Arial,加粗,四号,两端对齐,大纲级别2级,悬挂缩进1.02厘米,段前0行,段后0行,1.5倍行距,正规编号,起始编号阿拉伯数字“1”,编号位置左对齐,对齐位置0厘米,制表位位置1.02厘米,缩进位置1.02厘米。
三级标题:宋体+Arial,加粗,小四,两端对齐,大纲级别3级,悬挂缩进1.27厘米,段前0行,段后0行,1.5倍行距,正规编号,起始编号阿拉伯数字“1”,编号位置左对齐,对齐位置0厘米,制表位位置1.27厘米,缩进位置1.27厘米。
四级标题:宋体+Arial,加粗,五号,两端对齐,大纲级别2级,悬挂缩进1.52厘米,段前0行,段后0行,1.5倍行距,正规编号,起始编号阿拉伯数字“1”,编号位置左对齐,对齐位置0厘米,制表位位置1.52厘米,缩进位置1.52厘米。
万科金域传奇项目产品手册效果图目录一、总体规划篇二、建筑篇三、户型装修篇四、公共部位篇五、其它1.1 总体规划理念2.1 建筑风格及理念3.1 户型分布表4.1 地面大堂5.1 红线内不利因素1.2 建筑规划布局2.2 建筑外立面材料3.2 非标户型差异4.2 单体地上公共部位装修5.2 节能公示表1.3 景观规划布局2.3 建筑性能配置3.3 户型分析4.3 地下入户空间1.4 产品分布及指标 3.4 室内装修标准1.5 车行系统1.6 绿化围合一、规划景观篇总体规划篇1.1总体规划理念万科金域传奇位于宁波东南部,鄞州潘火街道。
地处东部新城、宁波老城区、鄞州南部新城三区交汇处,紧扼三区交通要塞。
总体规划理念方案重点营造内向型、具有吸引力、以高层住宅围合形成的小区中心环境。
总图平面营造出的居住空间模式,使居住于其中的人们一进入小区就宛如进入了一个公园,再回到自己温馨的家;本规划理念方案旨在为小区的居民们提供一个自然和谐的交往平台,使小区成为可开展各种家庭活动、社交活动的年轻时尚社区。
效果图主要技术经济指标项目数值计量单位规划净用地43811 平米面积计容净用地43752 平米面积总建筑面积123169.54 平米地上计容建100618.42 平米筑面积住宅户数946 户机动车位959 辆绿地率30 %建筑密度18.28 %容积率 2.3效果图小区人车行主要出入口(平时车辆双入)小区人车行次要出入口(平时车辆双出)地下机动车库出入口地下非机动车出入口 围墙(住宅) 围墙(幼儿园)3#5#6#7#8#2#1#4#前往规划公园人行出入口(规划公园为拟规划中)位置待定地下机动车库出入口(日常封闭) 备注:前往公园人行出入口为政府拟规划,是否存在以政府最终批准为准,此公园人行出入口不作为万科承诺。
效果图建筑间距1、高层建筑南北间距约41-68米。
2、5#楼与1、6#楼,6号楼与7号楼,7号楼与8号楼东西错开。
建筑层数18层25层3层 2#楼 商业(局部2层) 3、4、8#楼3层幼儿园(局部2层)效果图建筑朝向住宅朝向均为正南北33层 5、6、7#楼 26层 1#楼 3#4#8#2#1#7# 6#5#约41米 约68米约67米 约41米 约22米约25米住宅单元入户住宅单元入户方式均为北入户3#5#6#7#8#2#1#4#效果图景观总平面图绿地面积:约1,3125.6 m2绿地率:约30 %景观设计风格闹中取静,享受安宁健康的居住环境,是每个都市人的生活理想。
本项目的景观设计就是希望创造这样的生活空间:方案以自然为蓝本,把现代都市闲情文化融入到灵性的自然景观中,采用现代的营造手法,塑造出一个别致的自然风情园,运用自然质朴的材料,创造动人的居住艺术空间。
重新诠释现代人对园林式居住文化的理解。
在这个社区里,人们可以挥洒汗水,放纵活力,可以品茗观艺,赏花谈心,于一片祥和之地,凝固时光与回忆,细细品味诗意的主题。
效果图景观动区 Active Area主入口主出口商业街儿童游乐区中央广场景观静区 Quiet Area阳光草坪迷园效果图效果图效果图效果图效果图效果图效果图效果图效果图效果图户型 房型 面积 户数 A 三房两厅一卫 85-89 438 B三房两厅一卫 89 18 C 三房两厅一卫 89 66 D 两房两厅一卫 75 36 E三房两厅一卫106-110289F 三房两厅两卫 127 993#1#效果图7#4#8#6#5#户型配比楼号 层数 A B C DEF 合计(户) 1# 26 52 52 104 2# 25 50 50 100 3# 18 36 35 71 4# 18 36 35 71 5# 33 132 66 33 33 264 6# 33 66 66 132 7# 33 66 66 132 8# 18 18 36 18 72 合计438186636 289999462#商业(2、3F)住宅(18、25、26、33F)幼儿园(2、3F)商业局部有社区经营、社区管理、物业经营、物业管理、再生资源回收站等配套用房。
效果图商业社区经营用房物业管理用房再生资源回收站环网站一层北区二层北区商业平面图一层南区二层南区商业社区管理用房物业经营用房三层南区商业平面图1、2#楼标准层平面图(第6层)3#楼标准层平面图(第6层)1.5车行系统---围墙内车行道路道路规划与交通组织:本小区车行出入口设置在凤起北路及南侧规划道路上,车行道宽度约7米。
效果图总体规划篇车行道1.5车行系统---地面机动车停车位围墙内地面机动车停车位机动车位总计: 959辆。
包括地面机动车位与地下机动车位。
地面机动车位计293辆(其中幼儿园停车位7辆)效果图围墙外地面机动车停车位幼儿园地面机动车停车位总体规划篇围墙(住宅)围墙(幼儿园)1.5车行系统---地下机动车停车位机动车位总计: 959辆。
包括地面机动车位与地下机动车位。
地下机动车位计666辆,其中产权车位576辆,不可售人防车位90辆。
1#7#5#6#2#3#8#4#地下机动车库出入口 人防车位区域 地库采光井地下机动车库出入口(日常关闭) 总体规划篇效果图2#楼地下非机动车库坡道 5#楼地下非机动车库坡道4#楼地下非机动车库坡道除围墙外为地面非机动车停车外,其他均为地下非机动车库停车。
地下非机动车库分布于2#、4#、5#、6#、8#楼下。
6#楼地下非机动车库坡道 8#楼地下非机动车库坡道商业非机动停车(围墙外)2#楼地下自行车库非机动车区域地下非机动车出入口非机动车坡道1#7#5#6#2#3# 8#4#绿化围合图例•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
绿化围合开口备注:每个绿化围合中有1个约1m 宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
图例绿化围合绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
图例绿化围合绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
图例绿化围合绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
绿化围合图例绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
绿化围合图例绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
绿化围合图例绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
图例绿化围合绿化围合开口备注:每个绿化围合中有1个约1m宽绿化开口,此位置仅供示意,后期有可能调整,具体以实际交付为准。
•绿篱厚度约为0.5米•绿化围合范围内地面铺设草皮,绿化围合内可能会有雨污水井和弱电箱体等。
二、建筑篇主材——涂料(包括仿石涂料及真石漆、弹性涂料)辅材——石材(包括棕色和米黄色两种仅商业采用,黄色石材仅住宅单元门头采用)建筑篇2.1 建筑风格及理念建筑篇2.1 建筑风格及理念建筑外立面的设计采用现代国际风格,以质朴的材料与柔和的色彩表现清雅的格调;以体量错落来营造丰富的整体形象;以精雕细刻的细部设计来体现建筑的精美感。
住宅立面处理上,强调竖向线条以及纵伸感,使其具有一种明显的垂直性的竖向构图,使建筑更具挺拔感。
使其既保留浪漫主义风格的韵味,也不失现代感。
立面丰富而不繁琐,温馨耐看。
深棕色涂料深棕色涂料米黄色真石漆浅咖啡色仿石涂料3#楼南立面示意深棕色涂料米黄色真石漆内天井为弹性涂料浅咖啡色仿石涂料3#楼北立面示意米黄色真石漆浅咖啡色仿石涂料深棕色涂料深棕色涂料 6#楼北立面示意米黄色真石漆深棕色涂料浅咖啡色仿石涂料 6#楼南立面示意内天井为弹性涂料米黄色石材咖啡色铝板商业街立面示意深咖啡石材 备注:商业靠住宅面为涂料层数18层25、26层33层备注户型A、B、D、E A、E A、C、E、F结构形式剪力墙剪力墙剪力墙结构材料均为钢筋混凝土楼板厚度10cm(跨度较大区域局部有加厚)住宅客餐厅等区域局部楼板加厚,会对净高产生一定影响层高 2.85米 2.85米 2.85米此为结构层高,住宅装修完成后,净高以实际为准。
地下室层高非人防区3.3米,人防区3.4米此为结构层高,地下室净高一般高于2米墙体填充材料地上部分为砌块及砼,外墙及分户墙为20cm厚地上部分为砌块及砼,外墙及分户墙厚度为20cm及25cm厚样板房栏杆仅作样式参考,根据楼栋、楼层、单元不同略微有些差异保温系统内保温(局部外保温)安防住宅一、二层及顶层层数18层25、26层33层备注户型A、B、D、E A、E A、C、E、F电梯载重825/1050KG 载重为1050KG为担架电梯电梯数量一单元两台(825KG一台,1050KG一台)外立面门窗塑钢门窗;普通中空玻璃(8#楼东面、北面住宅为塑钢双层中空玻璃窗; 5#、6#、7#楼北面住宅为塑钢双层中空玻璃窗)住宅地上大堂单元门为不锈钢门,玻璃根据设计要求有所不同(5#、6#、7#、8#北面公共部位为普通中空玻璃)阳台栏杆铝合金玻璃栏杆或实体墙+铝合金玻璃栏杆样板房栏杆仅作样式参考,根据楼栋、楼层、单元不同略微有些差异设备平台栏杆铝合金方管栏杆样板房栏杆仅作样式参考,根据楼栋、楼层、单元不同略微有些差异楼梯栏杆钢管栏杆百叶铝合金百叶太阳能住宅逆六层设置(燃气热水器辅助加热)根据节能设计要求设置商业街性能配置结构形式框架层高一层4.4米,二层3.4米,三层3.4米此为结构层高门窗形式铝合金门窗及玻璃幕墙系统弱电系统中的电视、电话、网络配置备注D A 、B 、C 、EF餐厅电视墙一侧墙面 配置电视、网络模块(2合1型模块)客厅沙发墙一侧墙面 配置电话、网络模块(2合1型模块)、USB 充电插口主卧电视墙一侧墙面配置电视、网络模块(2合1型模块) 主卧床头一侧墙面配置电话、网络模块(2合1型模块)次卧电视墙一侧墙面配置电视模块书房/配置网络模块户型位置三、户型装修篇。