AMG 837 calcium hydrate_1259389-38-2_DataSheet_MedChemExpress
- 格式:pdf
- 大小:121.38 KB
- 文档页数:1
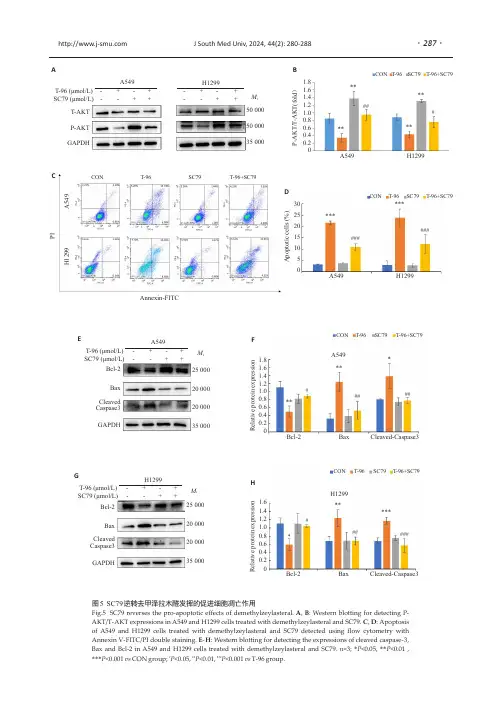
T-96(μmol/L)-+-+SC79(μmol/L)--++A549H1299M r 500005000035000T-AKT P-AKT GAPDH-+-+--++图5SC79逆转去甲泽拉木醛发挥的促进细胞凋亡作用Fig.5SC79reverses the pro-apoptotic effects of demethylzeylasteral.A ,B :Western blotting for detecting P-AKT/T-AKT expressions in A549and H1299cells treated with demethylzeylasteral and SC79.C ,D :Apoptosis of A549and H1299cells treated with demethylzeylasteral and SC79detected using flow cytometry with Annexin V-FITC/PI double staining.E -H :Western blotting for detecting the expressions of cleaved caspase-3,Bax and Bcl-2in A549and H1299cells treated with demethylzeylasteral and SC79.n =3;*P <0.05,**P <0.01,***P <0.001vs CON group;#P <0.05,##P <0.01,###P <0.001vs T-96group.T-96(μmol/L)-+-+SC79(μmol/L)--++A549Bcl-2Bax Cleaved Caspase3GAPDH25000200002000035000M r T-96(μmol/L)-+-+SC79(μmol/L)--++H1299M r 25000200002000035000Bcl-2Bax Cleaved Caspase3GAPDHCON T-96SC79T-96+SC79H1299Bcl-2Bax Cleaved-Caspase3R e l a t i v e p r o t e i n e x p r e s s i o n1.61.41.21.00.80.60.40.20######*****CON T-96SC79T-96+SC79A549Bcl-2Bax Cleaved-Caspase3R e l a t i v e p r o t e i n e x p r e s s i o n1.81.61.41.21.00.80.60.40.20#####*****EFGHCON T-96SC79T-96+SC79######******A549H1299A p o p t o t i c c e l l s (%)302520151050CON T-96SC79T-96+SC79P IH 1299A 549Annexin-FITCA549H1299P -A K T /T -A K T (f o l d )1.81.61.41.21.00.80.60.40.20CON T-96SC79T-96+SC79********###ABCD J South Med Univ,2024,44(2):280-288··287我们推测CREB 有可能是治疗NSCLC 的潜在有效靶点。

Design,Synthesis,and Biological Activity of Boronic Acid-Based Histone Deacetylase Inhibitors Nobuaki Suzuki,†Takayoshi Suzuki,*,†Yosuke Ota,†Tatsuya Nakano,‡Masaaki Kurihara,§Haruhiro Okuda,§Takao Yamori,# Hiroki Tsumoto,†Hidehiko Nakagawa,†and Naoki Miyata*,†,§Graduate School of Pharmaceutical Sciences,Nagoya City Uni V ersity,3-1Tanabe-dori,Mizuho-ku,Nagoya,Aichi467-8603,Japan,Di V ision of Medicinal Safety Science,National Institute of Health Sciences,1-18-1Kamiyoga,Setagaya-ku,Tokyo158-8501,Japan,Di V ision of Organic Chemistry,National Institute of Health Sciences,1-18-1Kamiyoga,Setagaya-ku,Tokyo158-8501,Japan,and Di V ision of Molecular Pharmacology,Cancer Chemotherapy Center,Japanese Foundation for Cancer Research,3-10-6Ariake,Koto-ku,Tokyo135-8550,Japan Recei V ed February2,2009Guided by the proposed catalytic mechanism of histone deacetylases(HDACs),we designed and synthesizeda series of boronic acid-based HDAC inhibitors bearing an R-amino acid moiety.In this series,compounds(S)-18,20,and21showed potent HDAC-inhibitory activity,highlighting the significance of the(S)-aminoacid moiety.In cancer cell growth inhibition assays,compounds(S)-18,20,and21exerted strong activity,and the values of the ratio of the concentration causing50%growth inhibition(GI50)to the concentrationcausing50%enzyme inhibition(IC50),i.e.,GI50/IC50,were low.The potency of these compounds was similarto that of clinically used suberoylanilide hydroxamic acid(SAHA)(2).The results of Western blot analysisindicated that the cancer cell growth-inhibitory activity of compounds(S)-18,20,and21is the result ofHDAC inhibition.A molecular modeling study suggested that the hydrated boronic acid interacts with zincion,Tyr residue,and His residue in the active site of HDACs.Ourfindings indicate that these boronic acidderivatives represent an entry into a new class of HDAC inhibitors.IntroductionThe acetylation status of lysine residues in nucleosomal histones is tightly controlled by two counteracting enzyme families,the histone acetyl transferases and the histone deacety-lases(HDACs a).1The latter family can be divided into two categories:Zn2+-dependent enzymes and NAD+-dependent enzymes.2The Zn2+-dependent HDACs are closely connected with control of gene expression and cell cycle progression.3The inhibition of HDACs causes histone hyperacetylation and leads to transcriptional activation of genes such as p21WAF/CIP1,Gadd 45,FAS,and caspase-3,which are associated with growth arrest and apoptosis in tumor cells.4Indeed,HDAC inhibitors,such as trichostatin A(TSA,1),5suberoylanilide hydroxamic acid (SAHA,also known as vorinostat,2),6PXD-101(3),7and MS-275(4)8(Chart1),have been reported to inhibit cell growth, induce terminal differentiation in tumor cells,and prevent the formation of malignant tumors,and they have been developed as antitumor agents.Among them,SAHA has recently been approved by the FDA for treatment of cutaneous T-cell lymphoma.In addition,there are several lines of evidence indicating that HDAC inhibitors are effective as therapeutic agents for other diseases,including inflammation and neuro-degenerative diseases.9Moreover,it has recently been reported that HDAC inhibitors,such as TSA(1),SAHA(2),and valproic acid,improve the efficiency of induction of pluripotent stem cells without introduction of the oncogene c-Myc,suggesting the importance of HDAC inhibitors in regenerative medicine.10Consequently,there are many ongoing research programs to find more potent and selective HDAC inhibitors.11To date,X-ray crystal structures of human HDAC4,12 HDAC7,13HDAC8,14and archaebacterial HDAC-like protein (HDLP)15have been published.A number of potent and selective inhibitors have been identified by structure-based drug design using these crystal structures.11Recently,the catalytic mechanism of the deacetylation of acetylated lysine substrates by HDACs was proposed,based on a density functional theory QM/MM study of HDLP,16and this also offers many clues for the design of HDAC inhibitors.Herein we report the synthesis and biological activity of boronic acid-based HDAC inhibitors, which were designed on the basis of the proposed catalytic mechanism of HDACs.ChemistryCompounds5-26prepared for this study are shown in Tables 1-3.The routes used for the synthesis of the compounds are shown in Schemes1-5.Scheme1shows the preparation of R-amino acid derivatives5-10.These compounds were syn-thesized from diethyl pound27was treated with(Boc)2O to yield N-Boc pound 28was allowed to react with5-bromopent-1-ene in the presence of NaOEt in refluxing EtOH to give C-alkylated compound29. Hydrolysis of the ethyl ester of29with an equivalent amount of LiOH gave monocarboxylic acid30,and subsequent decar-boxylation reaction afforded R-amino acid derivative31. Compound31was hydrolyzed with LiOH followed by treatment with an appropriate aromatic amine in the presence of EDCI and HOBt to give amides33-38.The hydroboration reaction of amides33-38with pinacolborane using[Ir(cod)Cl]2catalyst and dppm or dppe ligand gave terminal boronic esters39-44.17 Hydrolysis of the boronic esters39-44using NH4OAc and NaIO4in acetone-water yielded boronic acids5-10.18 Scheme2shows the preparation of boronic acids11-16,in which the R-amino moiety of compounds5-10was removed.*To whom correspondence should be addressed.For T.S.:phone andfax,+81-52-836-3407;e-mail,suzuki@phar.nagoya-cu.ac.jp.For N.M.:phone and fax,+81-52-836-3407;miyata-n@phar.nagoya-cu.ac.jp.†Nagoya City University.‡Division of Medicinal Safety Science,National Institute of HealthSciences.§Division of Organic Chemistry,National Institute of Health Sciences.#Japanese Foundation for Cancer Research.a Abbreviations:HDAC,histone deacetylase;TSA,trichostatin A;SAHA,suberoylanilide hydroxamic acid;HDLP,HDAC-like protein.J.Med.Chem.2009,52,2909–2922290910.1021/jm900125m CCC:$40.75 2009American Chemical SocietyPublished on Web04/14/2009These compounds were synthesized from hept-6-enoic acid 45using the same synthetic approach as described for compounds 5-10.Scheme 3shows the preparation of R -amino acid derivatives 17-24,in which the Boc group of 6was replaced with various carbonyl moieties.These compounds were prepared from 40in three steps:deprotection of the Boc group,condensation with an appropriate aromatic carboxylic acid,and hydrolysis of the boronic esters.Scheme 4shows the preparation of malonic acid derivatives 25and 26,in which the R -aminocarbonyl moiety of compounds 18and 20was replaced with the corresponding carboxamide.These compounds were synthesized from tert -butyl ethyl malonate 66.Treatment of 66with 5-bromopent-1-ene in the presence NaH in THF yielded compound 67.The ethyl ester of 67was hydrolyzed with an equivalent amount of LiOH,followed by condensation with biphenyl-3-ylamine to give amide 69.Hydroboration,deprotection of tert -butyl ester using TFA,condensation with an appropriate aromatic amine,and hydrolysis of the boronic ester gave the malonic acid derivatives 25and 26.Scheme 5shows the preparation of optically active R -amino acid derivatives (S )-and (R )-18,20,21.These compounds were synthesized using the procedure reported by Nishino and co-workers.19Compound (RS )-31was subjected to the action of subtilisin from Bacillus licheniformis in a mixture of DMF and water to yield (S )-32as a white solid.The recovered (R )-31was hydrolyzed with LiOH to afford (R )-32.The synthesis of (S )-and (R )-18,20,21was accomplished from (S )-32or (R )-32using the procedure described for the synthesis of R -amino acid derivatives (shown in Schemes 1and 3).The enantiomeric excess of (S )-and (R )-18,20,21was determined to be >95%by chiral column chromatography.Results and DiscussionDrug Design.In 1999,Finnin et al.reported X-ray crystal structures of HDLP complexed with inhibitors.15The enzyme contains a zinc ion at the bottom of the active site,and the active center consists of a tyrosine,two aspartic acids,and three histidines.In 2006,Corminboeuf et al.carried out density functional theory QM/MM studies on the deacetylation reaction catalyzed by HDLP.16The proposed mechanism suggested by the calculation results is depicted in Figure 1a.In this mecha-nism,the carbonyl oxygen of the substrate binds the zinc ion and is located adjacent to a water molecule.The electrophilicity of the carbonyl carbon is increased by coordination to the zinc ion,and so the carbonyl carbon is attacked by the water molecule activated by His 140and His 141(HDAC1number-ing).This nucleophilic attack results in a tetrahedral transition state,which is stabilized by a zinc -oxygen interaction and by hydrogen bonds with Tyr 303and His 140.In the final step,proton transfer from His 141to the nitrogen of the intermediate triggers scission of the carbon -nitrogen bond to afford the acetate and lysine products.In designing selective HDAC inhibitors,we focused on the proposed deacetylation mechanism.On the basis of this mech-anism,we designed R -amino acid derivatives A (Figure 1b)in which the acetamide of the acetylated lysine substrate is replaced by boronic acid.Because the boron atom of the boronic acids A has a vacant p-orbital,it could be attacked by a water molecule in the active site.The generated boronic acid -H 2O “ate”complex could act as a transition state analogue of the deacetylation of acetylated lysine substrates.That is,the hydrated boronic acid would bind the zinc ion and form two hydrogen bonds with Tyr 303and His 140,which would lead to HDAC inhibition.Enzyme Assays.In our exploratory study on boronic acid-based HDAC inhibitors,we initially focused on the R 1group (Table 1and Supporting Information Figure S1).We chose various aromatic rings as the R 1group because we had already discovered that thiolate compounds bearing aromatic groups such as phenyl,3-biphenyl,and 3-quinolinyl potently inhibited HDACs both in enzyme assays and in cellular assays.11BoronicChart 1.Examples of HDACInhibitorsScheme 1aaReagents and conditions:(a)(Boc)2O,Et 3N,THF,room temp,81%;(b)NaOEt,5-bromopent-1-ene,EtOH,reflux,89%;(c)LiOH ·H 2O,EtOH,H 2O,0°C,94%for 32,99%for 30;(d)toluene,reflux,94%;(e)R 1-NH 2,1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI),1-hydroxybenzo-triazole hydrate (HOBt ·H 2O),DMF,room temp,67-95%;(f)[Ir(cod)Cl]2,bis(diphenylphosphino)methane (dppm)or 1,2-bis(diphenylphosphino)ethane (dppe),pinacolborane,CH 2Cl 2,room temp,32-93%;(g)NH 4OAc,NaIO 4,acetone,H 2O,room temp,10-92%.2910Journal of Medicinal Chemistry,2009,Vol.52,No.9Suzuki et al.acids 5-10,in which R 1is various aromatic groups and R 2is -NHBoc,were initially tested with an in vitro assay using HeLa nuclear extract with high HDAC activity (Table 1and Figure S1).Compounds 5-10showed HDAC-inhibitory activity at a concentration of 100µM,while the 3-biphenyl 6,3-quinolinyl 7,and benzthiazole 8compounds were more potent (78-86%inhibition at 100µM).Compounds 6,7,and 8inhibited HDAC activity dose-dependently with IC 50values of 12,16,and 23µM,respectively;these values are much lower than those of the other aromatic analogues 5,9,and 10.We also tested compounds 11-16,which lack the NH-Boc group of com-pounds 5-10,and they were found to be much less potent inhibitors than compounds 5-10(Table 1and Figure S1),suggesting the significance of the R -amino acid moiety.To find more potent HDAC inhibitors,we next focused on the tert -butoxy group of compound 6,the most potent inhibitorScheme 2aaReagents and conditions:(a)R 2-NH 2,EDCI,HOBt,DMF,room temp,72-95%;(b)[Ir(cod)Cl]2,dppm or dppe,pinacolborane,CH 2Cl 2,room temp,13-74%;(c)NH 4OAc,NaIO 4,acetone,H 2O,room temp,35-94%.Scheme 3aaReagents and conditions:(a)HCl,AcOEt,CHCl 3,room temp,100%;(b)R 3-COOH,EDCI,HOBt,DMF,room temp,11-100%;(c)R 3-COCl,Et 3N,CH 2Cl 2,N,N -dimethyl-4-aminopyridine (DMAP),room temp,41%;(d)NH 4OAc,NaIO 4,acetone,H 2O,room temp,19-82%.Scheme 4aaReagents and conditions:(a)NaH,5-bromopent-1-ene,THF,reflux,52%;(b)LiOH ·H 2O,tert -BuOH,H 2O,0°C,100%;(c)biphenyl-3-ylamine,EDCI,HOBt,DMF,room temp,76%;(d)[Ir(cod)Cl]2,dppm,pinacolborane,CH 2Cl 2,room temp,70%;(e)TFA,room temp,100%;(f)(i)oxalyl dichloride,DMF,CH 2Cl 2,0°C;(ii)R 4-NH 2,Et 3N,CH 2Cl 2,0°C,29%for 71,51%for 72;(g)NH 4OAc,NaIO 4,acetone,H 2O,room temp,39%for 26,71%for 25.Boronic Acid-Based Inhibitors Journal of Medicinal Chemistry,2009,Vol.52,No.92911in Table 1.We initially changed the Boc group of compound 6to a Cbz group (compound 17)(Table 2and Supporting Information Figure S2).Compound 17exhibited about 3-fold higher potency than compound 6.We also examined the activity of compound 18,in which the benzyloxy group of compound 17was replaced with a simple benzene ring,and the activity of 18(IC 50) 2.0µM)was about 2-fold higher than that of compound 17.By the mimicking of the partial structure of TSA (1),we introduced the dimethylamino group at the 4-position of the phenyl ring of compound 18,but compound 19displayed a 6.5-fold decrease in potency compared to the parent compound 18.Next,we converted the phenyl ring of compound 18to various heteroaryl rings.The 2-indole 23and 2-quinoline 24compounds exhibited HDAC-inhibitory activity less potent than that of the phenyl compound 18,whereas the 3-pyridine 20,5-thiazole 21,and 4-thiazole 22derivatives showed potencies similar to or greater than that of the phenyl compound 18(IC 50values:20)2.0µM,21)1.4µM,22)4.7µM),highlighting the importance of small ring size.To confirm the importance of R -amino acid structure,we examined the activity of malonic acid derivatives 25and 26in which the R -aminocarbonyl moiety of compounds 18and 20is replaced by the corresponding carboxamide (Table 2and Figure S2).Compounds 25and 26showed about 15-and 25-fold decreases in potency compared with the parent compounds 18and 20,respectively.Scheme 5aaReagents and conditions:(a)subtilisin,H 2O/DMF (1:3),37°C,42%for (R )-31,50%for (S )-32;(b)LiOH ·H 2O,EtOH,H 2O,room temp,100%;(c)biphenyl-3-ylamine,EDCI,HOBt,DMF,room temp,56-65%;(d)[Ir(cod)Cl]2,dppm,pinacolborane,CH 2Cl 2,room temp,79-82%;(e)HCl,AcOEt,CHCl 3,room temp,100%;(f)R 5-COOH,EDCI,HOBt,DMF,room temp,64-90%;(g)NH 4OAc,NaIO 4,acetone,H 2O,room temp,4-87%.Figure 1.(a)Proposed mechanism for the deacetylation of acetylated lysine substrates.(b)Proposed mechanism of inhibition of HDACs by boronic acids A .2912Journal of Medicinal Chemistry,2009,Vol.52,No.9Suzuki et al.Next we examined the influence of optical -pounds (R )-18,20,21and (S )-18,20,21were prepared and evaluated for their inhibitory effects on HDAC1,HDAC2,HDAC6,and HDAC8,as well as total HDACs from nuclear extracts.As shown in Table 3and Supporting Information Figure S3,in all cases,(S )-18,20,and 21were at least 10-fold more active than (R )-18,20,and 21,respectively.Since the stereochemistry of (S )-18,20,and 21is the same as that of natural lysine,these results suggest that these boronic acids act in the active site of HDACs.Among (S )-18,20,and 21,(S )-21was found to be the most potent pound (S )-21inhibited all HDACs with IC 50values in the micromolar or submicromolar range.Although (S )-21was less potent than SAHA (2),the isoform inhibition profile of (S )-21was similar to that of 2,which is the only HDAC inhibitor currently used in clinical practice.Cellular Assays.To examine the effectiveness of boronic acid-based HDAC inhibitors as anticancer drugs and tools for biological research,compounds (S )-18,20,and 21,as well as SAHA (2),were tested in a cancer cell growth inhibition assay.For initial screening,we used stomach cancer MKN45cells because HDAC inhibitors have been reported to inhibit the cell growth of MKN45cells.20The results are summarized in Ta-ble 4.SAHA (2)and its derivatives have been reported to show potent HDAC-inhibitory activity in enzyme assays and cancer cell growth inhibition assays.21However,a relatively large shift in potency between enzyme assay and cellular assay (GI 50/IC 50,abbreviated as potency shift in this paper)was observed with those hydroxamate HDAC inhibitors.21Indeed,as shown in Table 4,SAHA (2)exhibited a large potency shift in the cancerTable 1.HDAC Inhibitory Activity of Boronic Acids 5-16aaValues are the mean of at least three experiments.IC 50of SAHA (2)is 0.28µM.Table 2.HDAC Inhibitory Activity of 17-26aaValues are the mean of at least three experiments.Table 3.HDAC Inhibitory Activity of (S )-and (R )-18,20,21aIC 50(µM)class Icompd HDAC (nuclear extract)HDAC1HDAC2HDAC8class II,HDAC620.280.350.25 1.70.028(S )-18 1.6>10b 3.6>100 1.1(R )-1817>100>100>10024(S )-20 1.4 2.80.83230.32(R )-2015>10099>10014(S )-210.92 2.20.53 6.60.11(R )-2124>100>100>1005.6aValues are the mean of at least three experiments.b 38%inhibition at 10µM.Table 4.Growth-Inhibitory Activity on MKN45Cells and GI 50/IC 50Values of SAHA (2)and (S )-18,20,21aGI 50/IC 50compd GI 50(µM)HDAC HDAC1HDAC227.3262129(S )-189.3 5.8<0.93 2.6(S )-20 5.5 3.9 2.0 6.6(S )-213.53.81.66.6aValues are the mean of two separate experiments.Boronic Acid-Based InhibitorsJournal of Medicinal Chemistry,2009,Vol.52,No.92913cell growth inhibition assay using MKN45cells.22The reason for the large potency shift of SAHA (2)is unclear,but it seems reasonable to assume that it is at least partly due to poor membrane permeability resulting from the highly polar character of hydroxamic ing Advanced Chemistry Development (ACD/Laboratories)software,version 8.14for Solaris,the log D (pH 7)values of methylhydroxamic acid (CH 3CONHOH)and methylboronic acid (CH 3B(OH)2)were calculated to be -1.59and 1.0,respectively.The calculation results suggest that boronic acid is more lipophilic than hydroxamic acid under physiological conditions,and boronic acid derivatives might permeate through the cell membrane more efficiently than hydroxamates;there-fore,they might not show such large potency shifts as hydroxamates like SAHA (2).We then evaluated the growth-inhibitory activity of (S )-18,20,and 21on MKN45cells.As we expected,(S )-18,20,and 21inhibited the growth of MKN45cells and showed smaller potency shift values than SAHA (2).Among (S )-18,20,and 21,compound (S )-21showed the highest activity,being more potent than SAHA (2).Next,we evaluated growth inhibition by SAHA (2)and compound (S )-21against nine human cancer cell lines (Table 5).Compound (S )-21exerted potent growth inhibition against various human cancer cells,with GI 50values ranging from 0.73to 9.1µM,and these inhibitory activities were similar to those of SAHA (2)(average GI 50of (S )-21is 4.0µM,that of SAHA is 6.0µM).In addition,compound (R )-21did not show strong activity against these cancer cell lines (Table S1and Figure S4in Supporting Information)with GI 50s values ranging from 13to 21µM,suggesting the responsibility of HDAC inhibition for cancer cell growth repression.Next,we performed Western blot analysis to examine the HDAC-inhibitory effects of boronic acid derivatives in cells.Since nuclear HDACs such as HDAC 1and HDAC2catalyze the deacetylation of histones,3d the acetylation level of histone H4in human colon cancer HCT116cells was analyzed after treatment of the cells with compounds (S )-18,20,and 21.As can be seen in Figure 2,the level of acetylated histone H4was elevated dose-dependently.The level of acetylated R -tubulin was also investigated because (S )-18,20,and 21displayed potent inhibition of HDAC6,which is reported to catalyze the deacetylation of R -tubulin.23As expected,compounds (S )-18,20,and 21caused a dose-dependent increase in acetylation of R -tubulin.These results suggest that the cancer cell growth inhibition by boronic acids (S )-18,20,and 21was the result of HDAC inhibition.Molecular Modeling.Since the results of the enzyme assays (Table 3)suggested that boronic acids act within the active center of HDACs,we studied the binding mode of hydrated (S )-21within this site.We calculated the low energy conforma-tion of the hydrated (S )-21complex docked in a model based on the crystal structure of HDAC8(PDB code 1T67)using Macromodel 9.6software (Figure 3).An inspection of the complex shows that one of the oxygen atoms of the hydrated boronic acid can coordinate to the zinc ion (distance between oxygen and zinc,2.04Å)(Figure 3,left).In addition,the hydrogen of the OH group of Tyr 306is located 1.95and 2.20Åfrom the two oxygen atoms of the hydrated boronic acid,Table 5.Growth Inhibition of Various Cancer Cells Using SAHA (2)and (S )-21aGI 50(µM)cell2(S )-21HBC-5breast cancer17 6.5SNB-78central nervous system 169.1HCT116colon cancer 0.580.73DMS114lung cancer 2.9 2.4LOX-IMVI melanoma 1.3 1.3SK-OV-3ovarian cancer 2.5 2.2RXF-631L renal cancer 2.0 3.2MKN45stomach cancer 7.3 3.5PC-3prostate cancer 4.6 6.7mean6.04.0aValues arethe mean of two separate experiments.Figure 2.Western blot detection of acetylated R -tubulin and histone H4levels in HCT116cells after 8h treatment with (S )-18,20,21and reference compound 2.Figure 3.View of the conformation of (S )-21(ball-and-stick)docked in the HDAC8catalytic core.Residues within 5Åfrom the zinc ion are displayed in the tube graphic (left),and the surface of the enzyme is displayed in gray (right).2914Journal of Medicinal Chemistry,2009,Vol.52,No.9Suzuki et al.which suggests that Tyr306can form two hydrogen bonds with the hydrated boronic acid.Moreover,a short distance(2.22Å) between one of the hydrogens of the hydrated boronic acid and the nitrogen of His142was also observed.This indicates that hydrated boronic acids inhibit HDACs by interacting with the zinc ion,Tyr residue,and His residue in the active center.On the surface of HDAC8,the biphenyl ring is located in the hydrophobic region formed by the benzene rings of Tyr100 and Phe152and the alkyl chain of Lys33,and the thiazole ring lies in the hydrophobic area formed by the benzene ring of Tyr100and the methylene groups of Asp101,Gly151,and Phe208(Figure3,right).It is also suggested that two hydrogen bonds can be formed between the two NH groups and the carboxylate anion of Asp101(the distances are1.72and1.74Å).The observed interactions between(S)-21and HDAC8on the surface of the enzyme suggest the importance of the amino acid structure and the(S)-configuration of the inhibitor. ConclusionOn the basis of the proposed catalytic mechanism for the deacetylation of acetylated lysine substrates by HDACs,we designed a series of boronic acids bearing an R-amino acid moiety as candidate mechanism-based HDAC inhibitors.Among the synthesized compounds,compounds(S)-18,20,and21 displayed potent HDAC-inhibitory activities,suggesting that these boronic acids act in the active site of pounds (S)-18,20,and21also showed cancer cell growth-inhibitory activities as potent as SAHA(2).Intracellular HDAC inhibition by compounds(S)-18,20,and21was confirmed by Western blot analysis of acetylated histone H4and acetylated R-tubulin.A molecular modeling study of the HDAC8/(S)-21complex suggested that the hydrated boronic acid interacts with zinc ion, Tyr residue,and His residue in the active site of HDACs. Thus,we have identified a novel lead structure from which it should be possible to develop more potent HDAC inhibitors. Although boronic acids have been used as inhibitors for various hydrolytic enzymes such as serine protease,24arginase,25and proteasome,26this is thefirst report of HDAC inhibitors containing a boronic acid moiety.The results obtained in this study suggest that boronic acid-based inhibitors of HDACs have considerable potential for the development of novel therapeutic agents and tools for biological research.Experimental SectionChemistry.Melting points were determined using a Yanagimoto micro melting point apparatus or a Bu¨chi545melting point apparatus and were left uncorrected.Proton nuclear magnetic resonance spectra(1H NMR)and carbon nuclear magnetic resonance spectra(13C NMR)were recorded with a JEOL JNM-LA500,JEOL JNM-A500,or Bruker Avance600spectrometer in solvents as indicated.Chemical shifts(δ)are reported in parts per million relative to the internal standard tetramethylsilane.Elemental analysis was performed with a Yanaco CHN CORDER NT-5analyzer,and all values were within(0.4%of the calculated values,which indicates>95%purity of the tested compounds.Fast atom bombardment(FAB)mass spectra were recorded on a JEOL JMS-SX102A mass spectrometer.In positive-mode MS analysis using 3-nitrobenzyl alcohol(NBA)as a matrix,boronic acids are esterified with NBA and the molecular weight detected corresponds to(M+2NBA-2H2O+H)+or(M+2NBA-2H2O)+.In negative-mode analysis using glycerol(Gly)as a matrix,the molecular weight detected corresponds to(M+Gly-2H2O-H)-.HPLC was performed with a Shimazu instrument equipped with a CHIRALPAK IA column(4.6mm×250mm,Daicel Chemical Industries),and the samples eluted at1mL/min with ethanol and n-hexane.Reagents and solvents were purchased from Aldrich,Tokyo Kasei Kogyo,Wako Pure Chemical Industries,and Kanto Kagaku and used without purification.Flash column chromatog-raphy was performed using silica gel60(particle size0.046-0.063 mm)supplied by Merck.6-tert-Butoxycarbonylamino-7-oxo-7-phenylaminoheptylbo-ronic Acid(5).Step1:Preparation of Diethyl2-tert-Butoxy-carbonylaminomalonate(28).To a suspension of diethyl ami-nomalonate(27;10.0g,47.2mmol)and Et3N(32.9mL,236mmol) in THF(20mL)was added a solution of(Boc)2O(20.6g,94.4 mmol)in THF(10mL),and the solution was stirred overnight at room temperature.The reaction mixture was poured into water and extracted with AcOEt.The AcOEt layer was washed with water and brine and dried over Na2SO4.Filtration and concentration in vacuo and purification by silica gelflash column chromatography (AcOEt/n-hexane)1/5)gave10.5g(81%)of28as a colorless oil:1H NMR(CDCl3,500MHz,δ,ppm)5.56(1H,d,J)6.7Hz), 4.95(1H,d,J)7.6Hz),4.27(4H,m),1.45(9H,s),1.30(6H,t, J)7.3Hz).Step2:Preparation of Diethyl2-tert-Butoxycarbonylamino-2-(penten-4-yl)malonate(29).To a solution of28(10.2g,37.2 mmol)obtained above in EtOH(15mL)was added NaOEt(2.78 g,40.9mmol)under Ar gas at0°C.The reaction mixture was stirred at0°C for7min.After that,to the solution was added 5-bromopent-1-ene(8.32g,55.8mmol),and the mixture was refluxed for10h.It was then poured into water and extracted with AcOEt.The AcOEt layer was washed with water and brine and dried over Na2SO4.Filtration and concentration in vacuo and purification by silica gelflash column chromatography(AcOEt/n-hexane)1/6)gave11.3g(89%)of29as a colorless oil:1H NMR (CDCl3,500MHz,δ,ppm)5.94(1H,broad s),5.76(1H,ddt,J) 17,10,6.4Hz),5.00(1H,dd,J)15,1.8Hz),4.95(1H,d,J)10 Hz),4.26-4.18(4H,m),2.28(2H,m),2.07(2H,q,J)7.3Hz), 1.43(9H,s),1.30-1.19(8H,m).Step3:Preparation of2-tert-Butoxycarbonylamino-2-(ethoxy-carbonyl)hept-6-enoic Acid(30).To a solution of29(11.3g,33.0 mmol)obtained above in EtOH/H2O(20mL/10mL)was added LiOH·H2O(1.52g,36.3mmol),and the solution was stirred overnight at0°C.The reaction mixture was neutralized with10% citric acid and extracted with AcOEt.The AcOEt layer was washed with brine and dried over Na2SO4.Filtration and concentration in vacuo gave10.3g(99%)of30as a yellow oil:1H NMR(CDCl3, 500MHz,δ,ppm)5.80-5.71(2H,m),5.01(1H,dd,J)17,1.5 Hz),4.97(1H,dd,J)10,1.8Hz),4.31-4.18(2H,m),2.22(2H, m),2.07(2H,m),1.44(9H,s),1.39-1.22(5H,m).Step4:Preparation of Ethyl2-tert-Butoxycarbonylamino-hept-6-enoate(31).A solution of30(10.3g,32.6mmol)obtained above in toluene(40mL)was refluxed for9h.The reaction mixture was concentrated and purified byflash column chromatography (AcOEt/n-hexane)1/6)to give8.51g(94%)of31as a yellow oil:1H NMR(CDCl3,500MHz,δ,ppm)5.77(1H,ddt,J)17, 10,6.7Hz),5.04-4.94(3H,m),4.28(1H,m),4.19(2H,m),2.07 (2H,m),1.81(1H,m,),1.63(1H,m),1.53-1.37(11H,m),1.28 (3H,t,J)7.3Hz).Step5:Preparation of2-tert-Butoxycarbonylaminohept-6-enoic Acid(32).To a solution of31(8.34g,30.8mmol)obtained above in EtOH/H2O(20mL/10mL)was added LiOH·H2O(1.25 g,29.8mmol),and the solution was stirred overnight at0°C.The reaction mixture was neutralized with10%citric acid and extracted with AcOEt.The AcOEt layer was washed with brine and dried over Na2SO4.Filtration and concentration in vacuo gave7.01g (94%)of32as a white solid:1H NMR(CDCl3,500MHz,δ,ppm) 5.78(1H,ddt,J)17,10,6.7Hz),5.06-4.94(3H,m),4.32(1H, m),2.09(2H,m),1.87(1H,m),1.68(1H,m),1.57-1.37(11H, m).Step6:Preparation of tert-Butyl1-(Phenylaminocarbonyl) hex-5-en-1-ylcarbamate(33).To a solution of32(1.00g,4.11 mmol)and aniline(560µL,6.17mmol)in DMF(15mL)were added1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochlo-ride(EDCI;1.18g,6.17mmol)and1-hydroxy-1H-benzotriazole monohydrate(HOBt·H2O;834mg,6.17mmol),and the mixture was stirred overnight at room temperature.The reaction mixtureBoronic Acid-Based Inhibitors Journal of Medicinal Chemistry,2009,Vol.52,No.92915。

SAFETY DATA SHEET1. IdentificationProduct identifierTomato Blossom Spray RTU Other means of identificationProduct code 32042Recommended use Agricutlural/ Horticultural Use- Foliar Fertilizer- Refer to product label Recommended restrictionsNone known.Manufacturer/Importer/Supplier/Distributor information Manufacturer Lawn and Garden Products, Inc.AddressPO Box 35000Company name Website Telephone Emergency Contact Number 1-559-994-9144Emergency phone numberCHEMTREC (24 hours):USA, Canada, Puerto Rico 1-800-424-3900E-mail Fresno, CA 937452. Hazard(s) identificationNot classified.Physical hazards Category 4Acute toxicity, oral Health hazardsCategory 2Skin corrosion/irritationCategory 2ASerious eye damage/eye irritationNot classified.Environmental hazards Not classified.OSHA defined hazardsLabel elementsSignal word WarningHazard statement Harmful if swallowed. Causes skin irritation. Causes serious eye irritation.Precautionary statementPreventionWash thoroughly after handling. Do not eat, drink or smoke when using this product. Wear protective gloves. Wear eye/face protection.ResponseIf swallowed: Call a poison center/doctor if you feel unwell. If on skin: Wash with plenty of water. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Specific treatment (see this label). Rinse mouth. If skin irritation occurs: Get medical advice/attention. If eye irritation persists: Get medical advice/attention. Take off contaminated clothing and wash before reuse.Storage Store away from incompatible materials.DisposalDispose of contents/container in accordance with local/regional/national/international regulations.Hazard(s) not otherwise classified (HNOC)None known.Supplemental information99.28% of the mixture consists of component(s) of unknown acute oral toxicity.3. Composition/information on ingredientsMixturesCAS number% Chemical name Common name and synonyms7664-38-2Phosphoric AcidOther components below reportable levels99.27692544710.7230745526*Designates that a specific chemical identity and/or percentage of composition has been withheld as a trade secret.4. First-aid measuresInhalation Move to fresh air. Call a physician if symptoms develop or persist.Skin contact Remove contaminated clothing. Wash with plenty of soap and water. If skin irritation occurs: Getmedical advice/attention. Wash contaminated clothing before reuse.Eye contact Immediately flush eyes with plenty of water for at least 15 minutes. Remove contact lenses, ifpresent and easy to do. Continue rinsing. Get medical attention if irritation develops and persists. Ingestion Rinse mouth. If vomiting occurs, keep head low so that stomach content doesn't get into the lungs.Get medical advice/attention if you feel unwell. Get medical attention if symptoms occur.Most importantsymptoms/effects, acute and delayed Symptoms may include stinging, tearing, redness, swelling, and blurred vision. May cause redness and pain. Severe eye irritation.Indication of immediate medical attention and special treatment needed Provide general supportive measures and treat symptomatically. Keep victim warm. Keep victim under observation. Symptoms may be delayed.General information Ensure that medical personnel are aware of the material(s) involved, and take precautions toprotect themselves. Show this safety data sheet to the doctor in attendance.5. Fire-fighting measuresSuitable extinguishing media Water fog. Foam. Dry chemical powder. Carbon dioxide (CO2).Unsuitable extinguishingmediaDo not use water jet as an extinguisher, as this will spread the fire.Specific hazards arising fromthe chemicalDuring fire, gases hazardous to health may be formed.Special protective equipmentand precautions for firefightersSelf-contained breathing apparatus and full protective clothing must be worn in case of fire.Fire-fightingequipment/instructionsMove containers from fire area if you can do so without risk.Specific methods Use standard firefighting procedures and consider the hazards of other involved materials. General fire hazards No unusual fire or explosion hazards noted.6. Accidental release measuresPersonal precautions, protective equipment and emergency procedures Keep unnecessary personnel away. Keep people away from and upwind of spill/leak. Wear appropriate protective equipment and clothing during clean-up. Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Ensure adequate ventilation. Local authorities should be advised if significant spillages cannot be contained. For personal protection, see section 8 of the SDS.Methods and materials for containment and cleaning up This product is miscible in water.Large Spills: Stop the flow of material, if this is without risk. Dike the spilled material, where this is possible. Cover with plastic sheet to prevent spreading. Absorb in vermiculite, dry sand or earth and place into containers. Following product recovery, flush area with water.Small Spills: Wipe up with absorbent material (e.g. cloth, fleece). Clean surface thoroughly to remove residual contamination.Never return spills to original containers for re-use. For waste disposal, see section 13 of the SDS.Environmental precautions Avoid discharge into drains, water courses or onto the ground.7. Handling and storagePrecautions for safe handling Do not taste or swallow. Avoid contact with eyes, skin, and clothing. Avoid contact with eyes. Avoidprolonged exposure. Provide adequate ventilation. Wear appropriate personal protectiveequipment. When using, do not eat, drink or smoke. Wash hands thoroughly after handling.Observe good industrial hygiene practices.Conditions for safe storage, including any incompatibilities Store in original tightly closed container. Keep container tightly closed. Store away from incompatible materials (see Section 10 of the SDS).8. Exposure controls/personal protectionOccupational exposure limitsUS. OSHA Table Z-1 Limits for Air Contaminants (29 CFR 1910.1000)Value Components TypePEL 1 mg/m3 Phosphoric Acid (CAS7664-38-2)US. ACGIH Threshold Limit ValuesValue Components TypeSTEL 3 mg/m3 Phosphoric Acid (CAS7664-38-2)TWA 1 mg/m3 US. NIOSH: Pocket Guide to Chemical HazardsValue Components TypeSTEL 3 mg/m3 Phosphoric Acid (CAS7664-38-2)TWA 1 mg/m3 Biological limit values No biological exposure limits noted for the ingredient(s).Appropriate engineering controls Good general ventilation (typically 10 air changes per hour) should be used. Ventilation rates should be matched to conditions. If applicable, use process enclosures, local exhaust ventilation, or other engineering controls to maintain airborne levels below recommended exposure limits. If exposure limits have not been established, maintain airborne levels to an acceptable level. Eye wash facilities and emergency shower must be available when handling this product.Individual protection measures, such as personal protective equipmentEye/face protection Face shield is recommended. Wear safety glasses with side shields (or goggles).Skin protectionHand protection Wear appropriate chemical resistant gloves.Other Wear appropriate chemical resistant clothing. Use of an impervious apron is recommended.Respiratory protection In case of insufficient ventilation, wear suitable respiratory equipment. Respiratory protection notrequired.Thermal hazards Wear appropriate thermal protective clothing, when necessary.General hygiene considerations Keep away from food and drink. Always observe good personal hygiene measures, such as washing after handling the material and before eating, drinking, and/or smoking. Routinely wash work clothing and protective equipment to remove contaminants.9. Physical and chemical properties Appearance Liquid.Physical state Liquid.Form Liquid.Color Colorless Odor Slight. Pungent Odor threshold Not available. pH 2.2Salt-Out / Crystallization Temp Not available. Melting point/freezing point Not available. Initial boiling point and boilingrangeNot available. Flash point Not available. Evaporation rate Not available. Flammability (solid, gas)Not available. Upper/lower flammability or explosive limits Flammability limit - lower(%)Not available.Flammability limit - upper(%)Not available.Explosive limit - lower (%)Not available.Explosive limit - upper (%)Not available.Vapor pressure 0.00001 hPa estimated Vapor density Not available.Relative density Not available.Solubility(ies)Solubility (water)Miscible Partition coefficient (n-octanol/water)Not available.Auto-ignition temperature Not available.Decomposition temperature Not available.ViscosityNot available.Other informationPercent volatile98.49 % estimated Pounds per gallon8.38 lb/gal typical10. Stability and reactivityReactivity The product is stable and non-reactive under normal conditions of use, storage and transport.Chemical stability Material is stable under normal conditions.Possibility of hazardous reactionsNo dangerous reaction known under conditions of normal use.Conditions to avoid Contact with incompatible materials.Incompatible materials Strong oxidizing agents.Hazardous decomposition productsNo hazardous decomposition products are known.11. Toxicological informationInformation on likely routes of exposureIngestionHarmful if swallowed.Inhalation Prolonged inhalation may be harmful.Skin contact Causes skin irritation.Eye contactCauses serious eye irritation.Symptoms related to thephysical, chemical andtoxicological characteristics Symptoms may include stinging, tearing, redness, swelling, and blurred vision. Skin irritation.Severe eye irritation. May cause redness and pain.Information on toxicological effectsAcute toxicity Harmful if swallowed. Not known.Test ResultsComponentsSpeciesPhosphoric Acid (CAS 7664-38-2)LD50Rabbit Dermal Acute 2740 mg/kg LD50RatOral 1530 mg/kg* Estimates for product may be based on additional component data not shown.Skin corrosion/irritation Causes skin irritation.Serious eye damage/eyeirritationCauses serious eye irritation.Respiratory or skin sensitizationRespiratory sensitizationNot available.Skin sensitizationThis product is not expected to cause skin sensitization.Germ cell mutagenicity No data available to indicate product or any components present at greater than 0.1% aremutagenic or genotoxic.Carcinogenicity This product is not considered to be a carcinogen by IARC, ACGIH, NTP, or OSHA.OSHA Specifically Regulated Substances (29 CFR 1910.1001-1050)Not listed.Reproductive toxicity This product is not expected to cause reproductive or developmental effects.Specific target organ toxicity -single exposureNot classified.Specific target organ toxicity -repeated exposureNot classified.Aspiration hazard Not available.Chronic effects Prolonged inhalation may be harmful.12. Ecological informationEcotoxicity The product is not classified as environmentally hazardous. However, this does not exclude thepossibility that large or frequent spills can have a harmful or damaging effect on the environment. Persistence and degradability No data is available on the degradability of this product.Bioaccumulative potential Not available.Mobility in soil No data available.Other adverse effects No other adverse environmental effects (e.g. ozone depletion, photochemical ozone creationpotential, endocrine disruption, global warming potential) are expected from this component. 13. Disposal considerationsDisposal instructions Collect and reclaim or dispose in sealed containers at licensed waste disposal site. Dispose ofcontents/container in accordance with local/regional/national/international regulations.Local disposal regulations Dispose in accordance with all applicable regulations.Hazardous waste code The waste code should be assigned in discussion between the user, the producer and the wastedisposal company.Waste from residues / unused products Dispose of in accordance with local regulations. Empty containers or liners may retain some product residues. This material and its container must be disposed of in a safe manner (see: Disposal instructions).Contaminated packaging Empty containers should be taken to an approved waste handling site for recycling or disposal.Since emptied containers may retain product residue, follow label warnings even after container isemptied.14. Transport informationDOTNot regulated as dangerous goods.IATANot regulated as dangerous goods.IMDGNot regulated as dangerous goods.15. Regulatory informationUS federal regulations This product is a "Hazardous Chemical" as defined by the OSHA Hazard CommunicationStandard, 29 CFR 1910.1200.All components are on the U.S. EPA TSCA Inventory List.This product is not known to be a "Hazardous Chemical" as defined by the OSHA HazardCommunication Standard, 29 CFR 1910.1200.TSCA Section 12(b) Export Notification (40 CFR 707, Subpt. D)Not regulated.CERCLA Hazardous Substance List (40 CFR 302.4)Phosphoric Acid (CAS 7664-38-2)Listed.SARA 304 Emergency release notificationNot regulated.OSHA Specifically Regulated Substances (29 CFR 1910.1001-1050)Not listed.Superfund Amendments and Reauthorization Act of 1986 (SARA)Hazard categories Immediate Hazard - YesDelayed Hazard - NoFire Hazard - NoPressure Hazard - NoReactivity Hazard - NoSARA 302 Extremely hazardous substanceNot listed.NoSARA 311/312 HazardouschemicalSARA 313 (TRI reporting)Not regulated.Other federal regulationsClean Air Act (CAA) Section 112 Hazardous Air Pollutants (HAPs) ListNot regulated.Clean Air Act (CAA) Section 112(r) Accidental Release Prevention (40 CFR 68.130)Not regulated.Not regulated.Safe Drinking Water Act(SDWA)US state regulationsUS. Massachusetts RTK - Substance ListPhosphoric Acid (CAS 7664-38-2)US. New Jersey Worker and Community Right-to-Know ActPhosphoric Acid (CAS 7664-38-2)US. Pennsylvania Worker and Community Right-to-Know LawPhosphoric Acid (CAS 7664-38-2)US. Rhode Island RTKPhosphoric Acid (CAS 7664-38-2)US. California Proposition 65WARNING: This product contains a chemical known to the State of California to cause cancer and birth defects or otherreproductive harm.International InventoriesCountry(s) or region Inventory name On inventory (yes/no)* Australia Australian Inventory of Chemical Substances (AICS)Yes Canada Domestic Substances List (DSL)Yes Canada Non-Domestic Substances List (NDSL)No China Inventory of Existing Chemical Substances in China (IECSC)Yes Europe European Inventory of Existing Commercial ChemicalYesSubstances (EINECS)Europe European List of Notified Chemical Substances (ELINCS)No Japan Inventory of Existing and New Chemical Substances (ENCS)No Korea Existing Chemicals List (ECL)No New Zealand New Zealand InventoryYes Philippines Philippine Inventory of Chemicals and Chemical SubstancesNo(PICCS)United States & Puerto Rico Toxic Substances Control Act (TSCA) InventoryYes *A "Yes" indicates that all components of this product comply with the inventory requirements administered by the governing country(s)A "No" indicates that one or more components of the product are not listed or exempt from listing on the inventory administered by the governingcountry(s).16. Other information, including date of preparation or last revisionIssue date07-21-2015Revision date07-22-2015Version #04Disclaimer Lawn and Garden Products cannot anticipate all conditions under which this information and itsproduct, or the products of other manufacturers in combination with its product, may be used. It isthe user’s responsibility to ensure safe conditions for handling, storage and disposal of theproduct, and to assume liability for loss, injury, damage or expense due to improper use. While theinformation contained herein are presented in good faith and believed to be accurate, it is providedfor your guidance only. Because many factors may affect processing or application, werecommend that you make tests to determine the suitability of a product for your particular purposeprior to use. No warranties of any kind, either expressed or implied, including warranties ofmerchantability or fitness for a particular purpose, are made regarding products described orinformation set forth, or that the products, or information may be used without infringing theintellectual property rights of others. In no case shall the information provided be considered apart of our terms and conditions of sale. Further, you expressly understand and agree that theinformation furnished by our company hereunder are given gratis and we assume no obligation orliability for the information given or results obtained, all such being given and accepted at your risk.。
琥珀酸脱氢酶(SDH )活性检测试剂盒说明书微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC0955 规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称 规格 保存条件 试剂一 液体110 mL×1瓶 -20℃保存 试剂二 液体0.6mL×2支 -20℃保存 试剂三 液体18 mL×1瓶 2-8℃保存 试剂四 液体3mL×1瓶 2-8℃保存 试剂五液体3mL×1瓶2-8℃保存溶液的配制:1、 试剂二:为易挥发试剂,用完后尽快密封,-20℃保存; 产品说明:SDH (EC 1.3.5.1)广泛存在于动物、植物、微生物和培养细胞中。
SDH 是线粒体的一种标志酶,位于线粒体内膜上的一种膜结合酶,是连接呼吸电子传递和氧化磷酸化的枢纽之一。
此外,为多种原核细胞产能的呼吸链提供电子。
SDH 催化琥珀酸脱氢生成延胡索酸,脱下的氢通过吩嗪二甲酯硫酸(PMS )传递还原2,6-二氯酚靛酚(DCPIP ),并且在600nm 处具有特征吸收峰,通过600nm 吸光度的变化,测定2,6-DCPIP 的还原速度,代表SDH 酶活性。
Succinic Acid + FAD Fumaric Acid + FADHFADH + PMS FAD + PMSH 2PMSH 2 + Dichlorophenolindophenol(600nm) PMS + Reduced Dichlorophenolindophenol 注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、水浴锅、台式离心机、可调式移液器、微量玻璃比色皿/96孔板、研钵/匀浆器、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1. 组织样本:称取约 0.1g 组织,加入1mL 试剂一和 10μL 试剂二,用冰浴匀浆器或研钵匀浆充分研磨,4℃11000g 离心10min ,取上清,置冰上待测。



Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AMG 837 calcium hydrate is a potent GPR40 agonist(EC50=13 nM) with a superior pharmacokinetic profile and robustglucose–dependent stimulation of insulin secretion in rodents.IC50 value: 13 nM (EC50) [1]Target: GPR40 agonistAMG 837 displayed the expected two–fold increase in potency on GPR4 (EC50 = 13 [±7] nM) compared to the racemic compound and its activity crossed over to the rat and mouse forms of GPR40 (EC50 = 23 and 13 nM, respectively). AMG 837 was found to be a partial agonist on GPR40 with maximal activity 85% of that shown by DHA under our standard assay conditions. AMG 837 is a highly potent stimulator of insulin secretion in MIN6 cells with an EC50 comparable to that seen in the aequorin Ca2+–flux assay. showedno significant activity in cell–based assays against PPARα, δ, and γ. An external panel of 64 receptors also revealed no significant activity with the exception of weak inhibition (IC50 = 3 uM) on the a2–adrenergic receptor. Overall, AMG 837 was both highly potent and selective in vitro.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [2]Cell membranes were prepared from an A9 cell line stably transfected with GPR40 (A9_GPR40). Cell membranes were mixed with various concentrations of AMG 837, 0.1 μM GDP, 400 pM [35S]–GTPγ in binding buffer (consisting of 20 mM Hepes pH 7.4, 100 mM NaCl and 5 mM MgCl2) in a volume of 200 μl/well. Plates were incubated for 60 minutes at room temperature. Next, 20 μl of 3%NP–40 were added to each well and the plates were further incubated for 30 minutes. This was followed by the addition of 20 μl of anti–Gq antibody (anti Gα q/11 antibody, 1:400 dilution) and the plates were incubated for an additional 60 min. Finally, 50 μl ofanti–rabbit–SPA beads were added to each well and the plates were incubated for 3 hrs. Antibody captured [35S]–GTPγ was measured using a Microbeta.Animal administration [2]AMG 837 was formulated for oral dosing using 1% methylcellulose (CMC), 1% Tween 80. For evaluation of AMG 837 following a single dose in rats, animals were fasted overnight and then randomized into dose groups based on their body weights. Thirty minutes after oral administration of their respective treatments, the animals received a 1 g/kg glucose challenge dose by intraperitoneal injection.Blood samples were collected at 0, 5, 15, 30, 60, and 120 minutes via tail vein after the glucose challenge. Glucose levels weremonitored with a Glucometer (Elite XL). Plasma insulin was measured using a rat insulin ELISA kit. For evaluation of AMG 837 in Zucker fatty rats, animals were randomized based on body weight and received either vehicle, 0.03 mg/kg, 0.1 mg/kg, or 0.3 mg/kg AMG 837once daily for 21 days by oral gavage. Treatments were administered between 0900 and 1000 h during the light cycle. On days 1 and 21, an intraperitoneal glucose tolerance test (IPGTT) was performed.Product Name:AMG 837 (calcium hydrate)Cat. No.:HY-13967B CAS No.:1259389-38-2Molecular Formula:C 26H 22F 3O 4-Molecular Weight:455.45Target:GPR40Pathway:GPCR/G Protein Solubility:DMSO: ≥ 42 mg/mLReferences:[1]. Houze JB, et al. AMG 837: a potent, orally bioavailable GPR40 agonist. Bioorg Med Chem Lett. 2012 Jan 15;22(2):1267–70.[2]. Lin DC, et al. AMG 837: a novel GPR40/FFA1 agonist that enhances insulin secretion and lowers glucose levels in rodents. PLoS One. 2011;6(11):e27270.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。

【巴索切削油】火花机油巴索电火花加工专用油Sorepi LM水溶性切削液Blasocut 2000 Universal Art.870Blasomill CMP 851/Blasomill CMP851Blasocut 2000 Universal SW Art.870-64Blaser Blasocut 2000 Universal-MD Art.870-20Blaser Basocut 2000 Universal Art.870-66lBlaser Blasocut 2000 CF-MD Art.875-20Blaser Blasocut 2000 CF SW Art.875-64Blaser Blasocut 2000 CF Art.875-66Blaser Blasocut 2000 Universal LF Art.870LFBlaser Blasocut 2000 Universal SW Art.870-64Blaser Blasocut 2000 SW Art.870SWBlaser Blasocut 4000 Strong Art.872-66Blaser Blasocut 4000 Strong SW Art.872-64Blaser Blasocut 4000 Strong SW Art.872SWBlaser Blasocut 4000 SW Art.872SWBlaser Blasocut 4000 Strong LF Art.872LFBlaser Blasocut 4000 Strong LF Art.872LFBlaser Blasocut Kombi Art.883-03Blaser Blasocut KombiBlaser Blasocut 2000 CF HDD Art.875-72Blasocut 2000 870-66Blasogrind 10 RZ巴索优质通用型磨削油Blasocut BC 25MDBlasocut 2000 CF-MD巴索通用型无氯切削液Grindex 10 巴索高性能全合成磨削液Vasco 1000巴索高性能切削液Grindex 10 CO 巴索高性能全合成磨削液Blasogrind HC 5巴索优质通用型磨削油Vascomill 42Blasocut BC 37 Mg巴索特殊型切削液Blasocut BC 35 Kombi SW巴索通用型切削液Blasocut BC 40 NF巴索高性能切削液Blasocut Kombi SW巴索水溶性的切削液Blasocut BC 20 SW巴索高性能切削液Blasocut BC 230通用型切削液Vascomill 22巴索高性能切削油Blasomill HD 10 CF巴索高性能矿物油基切削油Blasocut 2000 UniversalBlasogrind 10 RZ巴索优质通用型磨削油Blasogrind HC 10巴索优质通用型磨削油Blasocut 4000 Strong巴索高性能切削液Blasocut 2000 CF HDD巴索无氯切削液Blasocut 2000 Universal-MD巴索通用型切削液Synergy 15巴索高性能全合成切削液B-cool 645巴索半合成切削液Blasogrind HC 5巴索优质通用型磨削油油性切削油Blasomill HD 15 Art. 832Blasomill LCF 26Blasomill 10巴索高性能矿物油基切削油Vascomill 42 巴索高性能切削油Vascomill 22 巴索高性能切削油Vasco 1000巴索高性能切削液Blasomill CSF 10 巴索矿物油基切削油Blasomill VG 15•Blaser Blasogrin EX 300Blasogrin EX 300 XBlasomill LCF 16Blaser Blasomill LF 1Blaser Blasomill LF 2Blasomill CSF 22巴索矿物油基切削油Blasomill SK23Blasomill USK10Blaser Blasomill LCF 06Blasomill SK43Blaser Blasomill LF 2Blasomill SK13Blasomill LT46Blasomill LT32Blasomill LT22Blaser Blasomill LT10Blaser Blasomill LS 22Blaser Blasomill LF 4Blaser Blasomill LF 3Blasomill TLBBlaser Vascogrind SE 植物油基磨削油Blasomill 32巴索高性能矿物油基切削油Blasomill HD 15Blasomill HD 46巴索矿物油基非水溶性的切削液Blasomill CSF 22巴索矿物油基切削油Blasomill HD 10 CF巴索高性能矿物油基切削油Blasomill 10巴索高性能矿物油基切削油Blasomill 46巴索高性能矿物油基切削油乳化型切削液、磨削液Blasocut BC 20 SWBlasocut 2000 Universal SW全合成型切削液、磨削液浓缩剂...Synergy 15巴索(Blaser)切削油Blaser Grindex 10 CO 硬质合金专用磨削液 Art.1...Blasocut 2000 CF HDD 硬盘专用切削液 Art.875-7...Vascogrind SE 植物油基磨削油Blasomill HD CF 矿物油基无氯切削油巴索Blaser切削油产品总汇巴索Blaser切削油产品总汇巴索Blaser切削油产品总汇。

刘华文,邓美晴,陈北燕. QuEChERS-超高效液相色谱-串联质谱法测定茶叶中47种农药残留[J]. 食品工业科技,2024,45(9):255−264. doi: 10.13386/j.issn1002-0306.2023050213LIU Huawen, DENG Meiqing, CHEN Beiyan. Determination of 47 Pesticide Residues in Tea by QuEChERS Method Combined with Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry[J]. Science and Technology of Food Industry, 2024, 45(9):255−264. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2023050213· 分析检测 ·QuEChERS-超高效液相色谱-串联质谱法测定茶叶中47种农药残留刘华文1,邓美晴2,陈北燕3,*(1.广西-东盟食品检验检测中心,广西南宁 530029;2.广西医科大学,公共卫生学院,广西南宁 530022;3.南宁市卫生学校,广西南宁 530409)摘 要:建立一种QuEChERS-超高效液相色谱-串联质谱法同时测定茶叶中47种农药残留的分析方法。
样品经1%乙酸乙腈提取,QuEChERS 方法净化,配有电喷雾离子源(ESI±)的超高效液相色谱-串联质谱(UPLC-MS/MS ),在正负离子多反应监测(MRM )模式下同时测定,基质匹配外标法定量。
结果表明:47种目标化合物在一定范围内线性关系良好,相关系数(r )为0.9971~0.9996,检出限为0.001~0.006 mg/kg ,定量限为0.002~0.020 mg/kg ,平均回收率为73.4%~114.3%,相对标准偏差(n=6)为0.3%~19.9%。

Seppro®IgY 14 LC10 ColumnCatalog Number SEP040Storage Temperature 2–8 °CTECHNICAL BULLETINProduct DescriptionThe Seppro®IgY 14 Liquid Chromatography 10 (LC10) Column is based on avian antibody (IgY)-antigen interactions and optimized buffers for sample loading, washing, eluting, and column regeneration. The column is specifically designed to remove fourteen highly abundant proteins from human fluids such as serum or plasma. The following proteins are depleted in a single step:Albumin IgGα1-Antitrypsin IgAIgM TransferrinHaptoglobin α2-Macroglobulin Fibrinogen Complement C3α1-Acid Glycoprotein (Orosomucoid)HDL (Apolipoproteins A-I and A-II)LDL (mainly Apolipoprotein B)The targeted highly abundant proteins are simultaneously removed by the immobilized specific IgYs when crude biological samples are passed through the column.Selective immunodepletion provides an enriched pool of low abundance proteins for downstream proteomic analyses. Specific removal of these fourteen highly abundant proteins depletes ∼95% of the total protein mass from human serum or plasma. The low abundance proteins in the flow-through fractions can then be studied. Removal of highly abundant proteins enables improved resolution and dynamic range for one dimensional electrophoresis (1DGE), two dimensional (2DGE) electrophoresis, and liquid chromatography/ mass spectrometry (LC/MS). The collected flow-through fractions may need to be concentrated dependent upon the downstream application.Characteristics of the IgY 14 LC10 ColumnSize: 12.7 ×79.0 mm(10 ml bed volume)Capacity: 10.0 mg of total protein or ∼143 µl of human plasma based on an average protein concentration of 70 mg protein/ml.Note: If the protein concentration of the sample is unknown and the total serum protein level is potentially elevated, a reduction of the serum load to 100 µl is recommended for initial study to avoid potential abundant protein bleed through.Total protein mass removal: ∼95%Targeted depletion efficiency: 95% (average) Maximum operation pressure: 350 psi (21 bars) Antibody-modified resin only withstands 100 psi Flow rate: 0.5–2.0 ml/minuteOperating temperature: 18–25 °CShipping Buffer: 1×Dilution Buffer with 0.02% sodium azideColumn body materials: Polycarbonate column cylinder, Polyethylene frit, Tefzel®caps, Buna-N-rubberO-rings, Delrin®nut fittings, ETFE ferrules, andPTFE PFA tubing.Usage: Column may be used 100 times.2ComponentsSeppro IgY 14 LC10 Column 1 each (Catalog Number S5074)10×Dilution Buffer 3 ×200 ml Tris-Buffered Saline (TBS) -100 mMTris-HCl with 1.5 M NaCl, pH 7.4(Catalog Number S4199)10×Stripping Buffer 3 ×200 ml1 M Glycine, pH 2.5(Catalog Number S4324)10×Neutralization Buffer 3 ×80 ml1 M Tris-HCl, pH 8.0(Catalog Number S4449)Corning®Spin-X®Centrifuge Tube Filters 1 pack0.45 µm, pack of 100(Catalog Number CLS8163)Precautions and DisclaimerThis product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Specimen collection needs to utilize universal precautions.Preparation InstructionsPreparation of 1×concentration buffers -Separately dilute the three 10×buffers (Dilution, Stripping, and Neutralization Buffers) 10-fold with water. If precipitation occurs in the 10×buffers, allow the bottle to warm to room temperature and mix untilcompletely dissolved prior to use. Do not dilute all of the 10×Neutralization Buffer, save a volume of the 10×neutralization buffer for neutralization of eluted bound proteins if analysis of bound proteins is desired. Sample Preparation -It is not recommended to load unfiltered serum directly onto the column. Human serum samples should be diluted 5-fold with 1×Dilution Buffer. Samples may contain particulate materials, which can be removed with a 0.45 µm spin filter, centrifuge for 1 minute at 9,000 ×g.Storage/StabilityStore the column at 2–8 °C. After use, equilibrate the column with 1×Dilution Buffer containing 0.02% sodium azide and store the column at 2–8 °C with the end-caps tightly sealed. Do Not Freeze the column.ProcedureNote: Always use the three 1×buffers as the mobile phases for the LC procedure. Adjust the LC procedure appropriately for the instrumentation being used. Do not expose the column to solvents other than the three 1×buffers. Do not expose the column to organic solvents (like alcohols, acetonitrile, etc.), strong oxidizers, acids, or reducing agents and other protein denaturing agents (urea).1.Set up the three 1×buffers as the only mobilephases.2.Purge lines with the three 1×buffers and run the1×Dilution Buffer at 2 ml/minute without a columnto check the system back pressure.Note: The maximum operation pressure includesthe pressure introduced by the column and thesystem backpressure from the instrument. Usually, the pressure introduced by the column is less than50 psi. It is important to first check the systembackpressure of the instrument before using thiscolumn. If the system backpressure is more than300 psi, use tubing with a larger I.D.or change the flow-cell to tubing with a larger I.D. to reduce thesystem backpressure.3.Attach the column to chromatography instrument(see Appendix) and equilibrate it with1×DilutionBuffer for 20 minutes at a flow rate of 2.0 ml/minute to obtain a flat baseline.4.Set up a LC timetable (see Table 1) and run twomethod blanks by injecting 1,250µl of1×DilutionBuffer.Note: Adjust LC timetable based on instrumentation available, if necessary.5.Inject 1,250 µl of the diluted and filtered serum (seeSample Preparation), start with a flow rate of0.5 ml/minute for 30 minutes, wash the column at aflow rate of 2.0 ml/minute for 5 minutes, collectflow-through fraction, and store collected fractionsat –70 °C if not analyzed immediately.Note: Due to high salt concentration in the1×Dilution Buffer, buffer exchange of the flow-through fractions to a volatile buffer (for example,ammonium bicarbonate) is recommended prior tolyophilization.36.Elute bound proteins from the column with1×Stripping Buffer at a flow rate of 2.0 ml/minutefor 15 minutes and neutralize the eluted fractionswith 0.1×fraction volume of 10×Neutralizing Buffer.Note: Do not expose the column to the 1×Stripping Buffer for more than 20 minutes.7.Neutralize the column with 1×Neutralizing Buffer ata flow rate of 2.0 ml/minute for 10minutes.8.Re-equilibrate the column with 1×Dilution Buffer foran additional 10 minutes at a flow rate of2.0 ml/minute. The re-equilibrated column may bestored with 1×Dilution Buffer with 0.02% sodiumazide at 2–8 °C. Do Not Freeze the column.Table 1.Timetable for IgY 14 LC10 columnDetector Model: 166.ResultsFigure 1.Typical Depletion ChromatogramPeaks A & B aredepleted fractions.Peak C is boundfraction.ABC4AppendixTips for fitting column to most chromatography instruments1.Adapting the column (M6 fitting) to most HPLCsystems• Option 1: female M6 to male 10-32 one-piece adapter (Catalog Number 55069) -Most HPLCinstruments use 10-32 fittings with 1/16″tubing,so an adapter (one-piece fitting) can be used toconnect the column to the instrument. Thisinexpensive approach uses an adapter, a one-piece fitting with female M6 threading at oneend, to accept the male M6 fitting on the tubingconnected to the column, and 10-32 malethreading on the other end, to fit into thedetector or injector.Note: Use of this adapter will require removal ofthe fitting on the injector or detector.• Option 2: female M6/male 10-32 fitting tofemale 10-32/female 10-32 fitting two-pieceadapter (Catalog Number55068) –A two-pieceadapter can be used without changing thedetector or injector fittings on the HPLC. Again,the inexpensive approach is a two-pieceadapter. The female M6 end of the female/malefitting accepts the male M6 fitting from thecolumn, the male 10-32 end fits into one end ofthe female/ female 10-32 fitting. The other endof the female/female fitting accepts the 10-32fitting on the HPLC tubing.2.Adapting the column (M6 fitting) to non-metricmedium/low pressure liquid chromatographysystems• Option 1: female 1/4-28 to male 10-32 one-piece adapter (Catalog Number 55071) plus10-32 to female M6 two-piece adapter (CatalogNumber 55068) -Many medium/low pressuresystems use 1/4-28 fittings. An one-pieceadapter along with a two-piece adapter can beused to connect the column with M6 fittings tothese instruments. This method requires havingtubing with 1/4-28 fittings on the injector anddetector. These fittings will thread into thefemale 1/4-28 to male 10-32 fitting (CatalogNumber 55071). The male 10-32 will tread intothe two-piece adapter (Catalog Number 55068)that will then join to the M6 fitting.• Option 2: female 1/4-28 to female M6 one-piece adapter(Catalog Number 59259-U) -Like Option 1, this option requires the tubing onthe injector and detector already have male1/4-28 fittings. The male 1/4-28 fitting willthread into the adapter that will then join it tothe male M6 fitting.Note:Be sure to order two of these parts, onefor each end of the column.Troubleshooting GuideHigh backpressure -Clogged inlet frits may result in high backpressure, distorted peak shape, and diminished column lifetime. To prevent these problems, remove particulates from samples with a spin filter before loading.No bound fraction peak -Bound proteins can only be removed from the column by eluting with 1×Stripping Buffer. Check LC timetable to ensure enough column exposure time to the 1×Stripping Buffer for complete removal of bound proteins.Abnormal peak height -∼95% of serum/plasma proteins will be removed as the bound fraction. The peak height of the bound fraction is expected to be much greater than that of the flow-through fraction. If this order is reversed, two possibilities may be checked:• Column may not have been regenerated properly after previous use,resulting in lost capacity. Tocorrect this, elute bound proteins with 2 additionalcolumn volumes of 1×Stripping Buffer and thenneutralize and re-equilibrate the column with1×Neutralizing Buffer and 1×Dilution Buffer.• Check for signs of biological growth in the buffer reservoirs. Replace with fresh buffers for optimized column performance.Seppro is a registered trademark of Sigma-Aldrich®Biotechnology LP and Sigma-Aldrich Co.Corning and Spin-X are registered trademarks of Corning, Inc.Delrin and Tefzel are registered trademarks of E.I. du Pont de Nemours & Co., Inc.TD,KR,DEC,MAM,MJM 11/14-2©2014 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.。
高效液相色谱法测定甲基泼尼松龙琥珀酸钠含量乔晓芳【摘要】目的建立测定甲基泼尼松龙琥珀酸钠含量的高效液相色谱(HPLC)法.方法色谱柱采用Thermo C18柱(250 mm×4.0 mm,5μm),流动相为乙腈-3%冰醋酸(29:71),检测波长为254 nm,柱温为25℃,流速为1.0 mL/min,进样量为20μL.结果甲基泼尼松龙琥珀酸钠质量浓度在0.08~0.12 g/L范围内与峰面积线性关系良好(r=0.9995);平均回收率为99.16%,RSD为0.09%(n=5).结论该方法准确性高,重复性好,可用于甲基泼尼松龙琥珀酸钠原料药的质量控制.%Objective To establish an HPLC method for the content determination of methylprednisolone sodium succinate. Methods The mobile phase consisted of acetonitrile-3% glacial acetic acid(29 :71). The detection wavelength was 254 nm, the column temperature was 25 ℃ and the flow rate was 1. 0 mL/min. The sample volume was 20 μL. Results The linear relation of the concentration and peak area was good in the range of 0. 08-0. 12 mg/mL( r=0. 9995). The average percentage of recovery was 99. 16%, the RSD was 0. 09%( n=5). Conclusion The method is accurate and reproducible, which can be used for the quality control of methylprednisolone sodium succinate.【期刊名称】《中国药业》【年(卷),期】2017(026)005【总页数】3页(P29-31)【关键词】甲基泼尼松龙琥珀酸钠;高效液相色谱法;含量测定【作者】乔晓芳【作者单位】河南省食品药品审评查验中心,河南郑州 450000【正文语种】中文【中图分类】R927.2甲基泼尼松龙琥珀酸钠是甲基泼尼松龙琥珀酸钠针的原料药,临床上主要用于抗炎[1-2]、免疫抑制、休克[3-5]和内分泌失调的治疗。
Association for the Advancement of Medical Instrumentation协会中文名称:AAMI;美国医疗器械促进协会1 AAMI 7198-1998 (R 2004) CARDIOVASCULAR IMPLANTS - TUBUAL VASCULAR PROSTHESES-FDA RECOGNIZED2 AAMI 10993-1-2003 Biological evaluation of medical devices - Part 1: Evaluation and testing-FDA RECOGNIZED3 AAMI 10993-2-2006 Biological evaluation of medical devices - Part 2: Animal welfare requirements4 AAMI 10993-3-2003 Biological Evaluation of Medical Devices, Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity5 AAMI 10993-4-2002 Biological evaluation of medical devices - Part 4: Selection of tests for interaction with blood-Incorporates Amendment 1: 20066 AAMI 10993-5-1999 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 5: TEST FOR IN VITRO CYTOTOXICITY-FDA RECOGNIZED7 AAMI 10993-6-1995 (R 2001) BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 6: TESTS FOR LOCAL EFFECTS AFTER IMPLANTATION-FDA RECOGNIZED8 AAMI 10993-7-1995 (R 2001) Biological Evaluation of Medical Devices, Part 7: Ethylene Oxide Sterilization Residuals-Only FDA RECOGNIZED if used in conjunction to AAMI TIR19: 1988; Replaces AAMI ST29: 1988 and AAMI ST30: 1989 [Use: AAMI TIR19 AMD 1]9 AAMI 10993-9-1999 (R 2005) Biological evaluation of medical devices - Part 9: for identification and quantification of potential degradation products10 AAMI 10993-11-2006 Biological evaluation of medical devices - Part 11: Tests for systemic toxicity11 AAMI 10993-12-2002 Biological evaluation of medical devices - Part 12: Sample preparation and reference materials-FDA RECOGNIZED12 AAMI 10993-13-1999 (R 2004) Biological evaluation of medical devices, Part 13: Identification and quantification of degradation products from polymeric devices.13 AAMI 10993-14-2001 (R 2006) BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 14: IDENTIFICATION AND QUANTIFICATION OF DEGRADATION PRODUCT FROM CERAMICS14 AAMI 10993-15-2000 (R 2006) Biological evaluation of medical devices?Part 15: Identification and quantification of degradation products from metals and alloys15 AAMI 10993-16-1997 (R 2003) BIOLOGICAL EVALUATION OF MEDICAL DEVICES - PART 16: TOXICOKINETIC STUDY DESIGN FOR DEGRADATION PRODUCTS AND LEACHABLES FROM MEDICAL DEVICES16 AAMI 10993-17-2002 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART17: ESTABLISHMENT OF ALLOWABLE LIMITS FOR LEACHABLE SUBSTANCES17 AAMI 11135 ERTA-2005 Medical devices ?Validation and routine control of ethylene oxide sterilization-Erratum issued: 20 January 1995 (print copy only) and Erratum issued: 6 April 2005 (PDF copy only)18 AAMI 11135-1994 Medical devices - Validation and routine control of ethylene oxide sterilization-FDA RECGOGNIZED; esignations: AAMI OPEO, AAMI ST27, and AAMI TIR1 [Refer to: AAMI TIR14, AAMI TIR15, AAMI TIR16, AAMI TIR20, AAMI TIR28]19 AAMI 11137-1-2006 Sterilization of health care products - Radiation - Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices-Also replaces AAMI ST31, AAMI ST32, AAMI TIR520 AAMI 11137-3-2006 Sterilization of health care products - Radiation - Part 3: Guidance on dosimetric aspects-Also replaces AAMI ST31, AAMI ST32, AAMI TIR521 AAMI 11138-1-2006 Sterilization of health care products - Biological indicators - Part 1: General requirements [Refer to: AAMI 18472] 22 AAMI 11138-2-2006 Sterilization of health care products - Biological indicators - Part 2: Biological indicators for ethylene oxide sterilization processes [Refer to: AAMI 18472]23 AAMI 11138-3-2006 Sterilization of health care products - Biological indicators - Part 3: Biological indicators for moist heat sterilization processes [Refer to: AAMI 18472]24 AAMI 11138-4-2006 Sterilization of health care products - Biological indicators - Part 4: Biological indicators for dry heat sterilization processes [Refer to: AAMI 18472]25 AAMI 11138-5-2006 Sterilization of health care products - Biological indicators - Part 5: Biological indicators for low-temperature steam and aldehyde sterilization processes [Refer to: AAMI 18472]26 AAMI 11607-2-2006 Packaging for terminally sterilized medical devices - Part 2: Validation requirements for ing, sealing and assembly processes27 AAMI 11737-2-1998 Sterilization of Medical Devices - Microbiological Methods - Part 2: Tests of Sterility Per ed in the Validation of a Sterilization Process-FDA RECOGNIZED28 AAMI 13485-2003 Medical devices - Quality management systems - Requirements for regulatory purposes29 AAMI 14155-1-2003 Clinical investigation of medical devices for human subjects - Part 1: General requirements30 AAMI 14155-2-2003 Clinical investigation of medical devices for human subjects?Part 2: Clinical investigation plans31 AAMI 14160-1998 Sterilization of Single-Use Medical Devices Incorporating Materials of Animal Origin - Validation and Routine Control of Sterilization by Liquid Chemical Sterilants-FDA RECOGNIZED32 AAMI 14161-2000 Sterilization of health care products?Biological indicators?Guidance for the selection, use, and interpretation of results-FDA RECOGNIZED33 AAMI 14937-2000 Sterilization of health care products?General requirements for characterization of a sterilizing agent and the development, validation, and routine control of a sterilization process for medical devices-FDA RECOGNIZED34 AAMI 15223-2000 Medical Devices - Symbols to be used with medical device labels, labeling and in ation to be supplied.-FDA RECOGNIZED; Includes Amendment 1: 11/5/2001 and Amendment 2: 4/23/200435 AAMI 15225-2000 (R 2006) NOMENCLATURE - SPECIFICATION FOA A NOMENCLATURE SYSTEM FOR MEDICAL DEVICES FOR THE PURPOSE OF REGULATORY DATA EXCHANGE-Includes Amendment 1: 200436 AAMI 15674-2001 Cardiovascular implants and artificial organs —Hard shell cardiotomy/venous reservoir systems (with/without filter) and soft venous reservoir bags37 AAMI 15675-2001 Cardiovascular implants and artificial organs?Cardiopulmonary bypass systems?Arterial blood line filters38 AAMI 15882-2003 Sterilization of health care products - Chemical indicators - Guidance for selection, use, and interpretation of results-FDA RECOGNIZED39 AAMI 17665-1-2006 Sterilization of health care products - Moist heat - Part 1 Requirements for the development, validation and routine control of a sterilization process for medical devices- er Designation: AAMI ST2540 AAMI 25539-1 AMD 1-2005 Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses, Amendment 1: Test methods 41 AAMI 25539-1-2003 Cardiovascular implants?Endovascular devices?Part 1: Endovascular prostheses42 AAMI 60601-2-21-2000 Medical electrical equipment, Part 2: Particular requirements for the safety of infant radiant warmers-FDA RECOGNIZED; er Designation: AAMI II52; Incorporates Amendment 1: 2000 43 AAMI 62304-2006 Medical device software - Software life cycle processes [Refer to: AAMI TIR32]44 AAMI BE78-2002 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 10: TEST FOR IRRITATION AND DELAYED TYPE HYPERSENSITIVITY-In place of AAMI 10993-10; FDA RECOGNIZED45 AAMI BE83-2006 Biological evaluation of medical devices - Part 18: Chemical characterization of materials46 AAMI BF7-1989 (R 2002) Blood Transfusion Micro-Filters-FDA RECOGNIZED47 AAMI BF64-2002 LEUKOCYTE REDUCTION FILTERS48 AAMI 7199-1996 (R 2002) Cardiovascular implants and artificial organs - Blood-ga* **changers (oxygenators)49 AAMI BP22-1994 (R 2006) Blood Pressure Transducers-FDA RECOGNIZED; Incorporates Errata: 08/05/200450 AAMI 5840-2005 Cardiovascular implants - Cardiac valve prostheses-FDA RECOGNIZED51 AAMI DF80-2003 Medical electrical equipment, Part 2: Particular requirements for the safety of cardiac defibrillators [including automated external defibrillators]-Also repalces AAMI TIR252 AAMI EC11-1991 (R 2001) Diagnostic electrocardiographicdevices-FDA RECOGNIZED53 AAMI EC12-2000 (R 2005) Disposable ECG Electrodes-FDA RECOGNIZED54 AAMI EC13 ERTA-2002 Cardiac Monitors, Heart Rate Meters and Alarms55 AAMI EC13-2002 Cardiac Monitors, Heart Rate Meters and Alarms56 AAMI EC38-1998 Ambulatory Electrocardiographs-FDA RECOGNIZED57 AAMI EC53 ERTA-2001 ECG cables and leadwires-Includes Erratum issued: 31 May 199858 AAMI EC53-1995 (R 2001) ECG cables and leadwires59 AAMI EC57-1998 (R 2003) Testing and reporting per ance results of cardiac rhythm and ST segment measurement algorithms-Includes Errata: 4/14/2004; Revision of ECAR: 1987; FDA-Recognized60 AAMI EC71-2001 Standard Communications Protocol - Computer Assisted Electrocardiography-Proposed equivalency to IEC60601-2-53/Ed.1: Harmonized61 AAMI EQ56-1999 (R 2004) Recommended practices for a medical equipment management program62 AAMI ES60601-1-2005 Medical electrical equipment, Part 1: General requirements for basic safety and essential per ance63 AAMI HE48-1993 Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices64 AAMI HE74-2001 Human Factors Design Process for MedicalDevices-FDA RECOGNIZED65 AAMI HFMD-1996 An Introduction to Human Factors in Medical Devices66 AAMI ID26 ERTA-2006 Medical electrical equipment, Part 2: Particular requirements for the safety of infusion pumps and controllers 67 AAMI ID54-1996 (R 2005) Enteral Feeding Set Adapters and Connectors-FDA RECOGNIZED68 AAMI NS4-1986 (R 2002) Transcutaneous Electrical Nerve Stimulators69 AAMI NS14-1995 (R 2002) Implantable spinal cord stimulators70 AAMI NS15-1995 (R 2002) Implantable peripheral nerve stimulators71 AAMI NS28-1988 (R 2006) Intracranial Pressure Monitoring Devices-FDA RECOGNIZED; Incorporates Errata: 06/200172 AAMI PAC49-1993 (R 2000) Pacemaker Emergency Intervention System73 AAMI PB70-2003 LIQUID BARRIER PER ANCE AND CLASSIFICATION OF PROTECTIVE APPAREL AND DRAPES INTENDED FOR USE IN HEALTH CAREFACILITIES-FDA RECOGNIZED74 AAMI PC69-2000 ACTIVE IMPLANTABLE MEDICAL DEVICES ELECTROMAGNETIC COMPATIBILITY EMC TEST PROTOCOLS FOR IMPLANTABLE CARDIAC PACEMAKERS AND IMPLANTABLE CARDIOVERTER DEFIBRILLATORS75 AAMI RD5-2003 Hemodialysis Systems76 AAMI RD16 AMD 1-2002 (R 2005) Hemodialyzers-FDA RECOGNIZED77 AAMI RD16-1996 (R 2005) Hemodialyzers [Refer to: IEC 60601-2-16]78 AAMI RD17-1994 (R 2005) Hemodialyzer Blood Tubing-Includes Amendment 1: 200279 AAMI RD47-2002 Reuse of Hemodialyzers-Incorporates Amendment 1: 7/2003 and Errata: 07/200380 AAMI RD52-2004 Dialysate for hemodialysis81 AAMI RD62-2006 WATER TREATMENT EQUIPMENT FOR HEMODIALYSIS APPLICATIONS82 AAMI SP10-2006 Manual, electronic, or automated sphygmomanometers-FDA REGOGNIZED; Incorporates Amendment 1: 2003; Amendment 2: 200683 AAMI ST8-2001 Hospital Steam Sterilizers-FDA RECOGNIZED84 AAMI ST24-1999 (R 2005) Automatic, General-Purpose Ethylene Oxide Sterilizers and Ethylene Oxide Sterilant Sources Intended for Use in Health Care Facilities-FDA RECONIZED85 AAMI ST40-2004 Table-top dry heat (heated air) sterilization and sterility assurance in health care facilities-FDA RECOGNIZED86 AAMI ST41-1999 (R 2005) Ethylene Oxide Sterilization in Health Care Facilities: Safety and Effectiveness-FDA RECOGNIZED87 AAMI ST50-2004 Dry Heat (Heated Air) Sterilizers-FDA RECOGNIZED88 AAMI ST55-2003 Table-Top Steam Sterilizers-FDA RECOGNIZED89 AAMI ST63-2002 Sterilization of health care products—Requirements for the development, validation, and routine control of an industrial sterilization process for medical devices—Dry heat-FDA RECOGNIZED90 AAMI ST65-2000 PROCESSING OF REUSABLE SURGICAL TEXTILES FOR USE IN HEALTH CARE FACILITIES91 AAMI ST66-1999 STERILIZATION OF HEALTHCARE PRODUCTS - CHEMICAL INDICATORS - PART 2 : CLASS 2 INDICATORS FOR AIR REMOVAL TEST SHEETS AND PACKS-FDA RECOGNIZED [Refer to: AAMI 18472]92 AAMI ST67-2003 Sterilization of health care products—Requirements for products labeled “STERILE?FDA RECOGNIZED93 AAMI ST77-2006 Containment devices for reusable medical device sterilization94 AAMI ST81-2004 Sterilization of medical devices - In ation to be provided by the manufacturer for the processing of resterilizable medical devices-FDA RECOGNIZED95 AAMI TIR4-1989 Apnea Monitoring by Means of Thoracic ImpedancePneumography96 AAMI TIR11-2005 Selection and use of protective apparel and surgical drapes in health care facilities97 AAMI TIR12-2004 Designing, Testing and Labeling Reusable Medical Devices for Reprocessing in Health Care Facilities: A Guide for Device Manufacturers-2nd Edition98 AAMI TIR13-1997 Principles of Industrial Moist HeatSterilization-(Updated material from ANSI/AAMI ST25:1987 )99 AAMI TIR14-1997 Contract Sterilization for EthyleneOxide-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994; Includes Amendment 1: 2004 [Refer to: AAMI 11135 ERTA]100 AAMI TIR15-1997 Ethylene Oxide Sterilization Equipment, Process Considerations and Pertinent Calculations-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]101 AAMI TIR16-2000 PROCESS DEVELOPMENT AND PER ANCE QUALIFICATION FOR ETHYLENE OXIDE STERILIZATION - MICROBIOLOGICAL ASPECTS-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" forANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]102 AAMI TIR17-1997 Radiation Sterilization MaterialQualification-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11137: 1994; Replaces AAMI ST32 [Refer to: AAMI 11137]103 AAMI TIR18-1997 Guidance on Electromagnetic Compatibility of Medical Devices for Clinical/Biomedical Engineers - Part 1: Radiated Radio-Frequency Electromagnetic Energy [Replaced by: IEC 61326-2-6] 104 AAMI TIR19 AMD 1-1999 Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological evaluation of medical deices—Part 7: Ethylene oxide sterilization residuals Amendment105 AAMI TIR19-1998 Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological Evaluation of Medical Devices - Part 7: Ethylene Oxide Sterilization Residuals-Replaces AAMI ST29 and AAMI ST30; Cited as relevant guidance to FDA-recognized standard ANSI/AAMI/ISO 10993-7 [Refer to: AAMI 10993-7]106 AAMI TIR20-2001 PARAMETRIC RELEASE FOR ETHYLENE OXIDE STERILIZATION-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]107 AAMI TIR21-1998 Systems Used to Forecast Remaining Pacemaker Battery Service Life108 AAMI TIR22-1998 Guidance for ANSI/AAMI/ISO 11607, Packaging for Terminally Sterilized Medical Devices-AMD 1: 2001109 AAMI TIR23-1999 Signal Averaging110 AAMI TIR24-1999 Acquisition and use of physiologic wave databases for testing of medical devices111 AAMI TIR26-2000 Ventricular assist and heart replacement systems 112 AAMI TIR28-2001 PRODUCT ADOPTION AND PROCESS EQUIVALENCY FOR ETHYLENE OXIDE STERILIZATION [Refer to: ISO 11135, AAMI 11135 ERTA] 113 AAMI TIR29-2002 GUIDE FOR PROCESS CONTROL IN RADIATION STERILIZATION-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11137: 1994 [Refer to: AAMI 11137]114 AAMI TIR30-2003 A Compendium of processes, materials, test methods, and acceptance criteria for cleaning reusable medical devices 115 AAMI TIR31-2003 PROCESS CHALLENGE DEVICES/TEST PACKS FOR USE IN HEALTH CARD FACILITIES116 AAMI TIR32-2004 Medical device software risk management [Refer to: AAMI 14971, AAMI 62304]117 AAMI TIR35-2006 Sterilization of health care products - Radiation sterilization - Alternative sampling plans for verification dose experiments and sterilization dose audits- er designation: AAMI ST31, AAMI ST32, and AAMI TIR5118 AAMI TIR10993-19-2006 Biological evaluation of medical devices - Part 19: Physico-chemical, morphological and topographical characterization of materials119 AAMI TIR10993-20-2006 Biological evaluation of medical devices - Part 20: Principles and methods for immunotoxicology testing of medical devices120 AAMI TIR11139-2006 Sterilization of health careproducts ?Vocabulary121 AAMI TIR14969-2004 Medical devices—Quality management systems?Guidance on the application of ISO 13485:2003122 AAMI TIR15844-1998 Sterlization of Health Care Products - Radiation Sterlization - Selection of a Sterilization Dose for a Single Production Batch-Edition 1123 AAMI TIR16142-2005 Guidance on the selection of standards in support of recognized essential principles of safety and per ance of medical devices-2006 printing124 AAMI TIR19218-2006 Medical devices - Coding structure for adverse event type and cause125 AAMI TIR60878-2003 Graphical symbols for electrical equipment in medical practice126 AAMI TIR62296-2003 Considerations of unaddressed safety aspects in the Second Edition of IEC 60601-1 and proposals for new requirements [Refer to: IEC 60601-1]127 AAMI TIR62348-2006 Mapping between the clauses of the third edition of IEC 60601-1 and the 1988 edition as amended。
高效液相色谱-质谱/质谱法测定猪肉中11种磺胺类药物残留发布时间:2022-08-01T08:38:02.400Z 来源:《科学与技术》2022年第6期作者:许计元刘淑梅李梦平[导读] 利用高效液相色谱-质谱/质谱仪建立猪肉中11种磺胺类药物残留的检测方法。
许计元,刘淑梅,李梦平安徽创佳安全环境科技有限公司利用高效液相色谱-质谱/质谱仪建立猪肉中11种磺胺类药物残留的检测方法。
本方法用乙腈作为提取溶剂,正己烷去除油脂,液相初始流动相定容,0.1%甲酸水与纯乙腈作为流动相,使用的色谱柱为安捷伦InfinityLab Poroshell 120 EC C18(3.0mm×150mm,2.7μm),多重反应监测(MRM)模式下采集数据,基质配标法定量。
11种磺胺类药物在1ug/kg-10ug/kg范围内线性良好,在4ug/kg加标水平下,回收率在86.9%-103.6之间,相对标准偏差在1.35%-3.28%之间。
本方法前处理简单,回收率、精密度与准确度均可以满足使用要求。
关键词:液相色谱;质谱;猪肉;磺胺Determination of 11 sulfonamides residues in pork by high performance liquid chromatography-mass spectrometry/mass spectrometryXu Ji-Yuan,Liu Shu-Mei,Li Meng-Ping(Anhui Chuangjia Safety environment Technology Co., LTD)A method for the determination of 11 sulfonamides residues in pork was established by high performance liquid chromatography-mass spectrometry. The chromatographic column was agilent InfinityLab Poroshell 120 EC C18(3.0mm×150mm,2.7μm), using acetonitrile as the extraction solvent, n-hexane for oil removal, and 0.1% formic acid water as the mobile phase. Data were collected in multiple response monitoring (MRM) mode and quantified by matrix coordination method. The linearity of 11 sulfonamides was good in the range of 1ug/kg-10ug/kg. At the spiked level of4ug/kg, the recoveries were 86.9%-103.6, and the relative standard deviations (RSDS) were 1.35%-3.28%. The method is simple in pretreatment, and the recovery, precision and accuracy can meet the application requirements.Key words: liquid chromatography; Mass spectrometry; Pork; Conditions磺胺类药物(SAs)是指具有对氨基苯磺酰胺结构药物的总称,为人工合成的抗菌药,该类药物具有抗菌谱广、抗菌效果明显、性质稳定、价格便宜等优点, 目前大量用于防治畜禽细菌性感染疾病。
HELIFAB1318 SMEDE HWY.BROUSSARD, LA 70518REPORT NUMBERHF-206-ICA-OXYGEN BOTTLE MOUNT-1THIS MANUAL IS PREPARED TO PROVIDE INFORMATION, INSTRUCTIONS FOR CONTINUED AIRWORTHINESS, MAINTENANCE INSTRUCTIONS AND REPAIR PROCEDURES FOR EQUIPMENT MANUFACTURED BY HELIFAB THAT MAY BE INSTALLED ON THE 206 HELICOPTER. ENSURE THAT THIS MANUAL IS USED FOR ONLY HELIFAB EQUIPMENT.TABLE OF CONTENTSITEM PAGETITLE PAGE 1 TABLE OF CONTENTS 2 RECORD OF REVISIONS 3Chapter 1 - INTRODUCTION1.1 Introduction 41.2 General 51.3 Consumable Materials 5Chapter 4 - AIRWORTHINESS LIMITATIONS4.1 Time Change Items 6Chapter 5 - TIME LIMITS/INSPECTIONS5.1 Conditional Inspections after Operational Incidents 75.2 Preflight Check – Equipment 75.3 100 Hour Inspection 75.4 12 Months/1200 Hour Inspection 8 Chapter 6 - WEIGHT AND BALANCE6.1 Weight and Balance 9CHAPTER 35 - MEDICAL EQUIPMENT35.1 Introduction 10 35.2 General Description 10 35.3 Removal/Installation/Troubleshooting –oxygen bottle mount 14 35.4 Removal/Installation - Decal 14 35.5 Cleaning 14 35.6 Repair 14 FIGURES TITLE PAGEFIGURE 1 Oxygen Bottle Mount Primary Installation Location 11 FIGURE 2 Oxygen Bottle Mount Alternate Installation Location 11 FIGURE 3 Oxygen Bottle Mount Installation 12 FIGURE 4 Section C-C 13 FIGURE 4a Section C-C 13RECORD OF REVISIONSCHAPTER 1 - INTRODUCTION1.1 IntroductionA. Scope of the aircraft maintenance manual.(1) The equipment maintenance manual Document No. HF-206-ICA-OXYGEN BOTTLEMOUNT-1)describes the required procedures for maintaining continued airworthinessfor the Bell 206 L series aircraft with the HeliFab oxygen bottle mount installation.This manual only includes information for servicing, maintenance,inspection and repair within the scope of an appropriately rated mechanicor repair station.B. Changes to the Equipment Maintenance Manual:(1) The current revision of this ICA may be obtained online at It is the responsibility of the owner/ operator that only the currentissue of the Equipment Maintenance Manual is used.(2) Changes to the equipment maintenance manuals are to be incorporated.(3) Changes are identified as follows:(a) Revised or extended text, inserted pages with new text contentsor new figures are identified with black marginal bars orreferenced symbols.(b) When text is relocated, resulting possibly in a renumbering ofpages or task, or in case of printing error corrections, nomarkings are provided.(c) Changed pages are provided with the issue date of the change.(4) Equipment manufacturer’s manuals;-Upon initial installation of the HeliFab oxygen bottle mount installation, HeliFab will provide any installation or operators manuals that are included with the purchase ofthe new equipment.C. Items of special emphasisWARNING IS USED WHEN UNQUALIFIED PERFORMANCE ORNEGLECT OF INSTRUCTIONS MAY LEAD TO INJURIESOR DEADLY ACCIDENTS.CAUTION IS USED WHEN UNQUALIFIED PERFORMANCE ORNEGLECT OF INSTRUCTIONS MAY LEAD TO EQUIPMENTDAMAGENOTE IS USED WHEN A PARTICULAR ITEM NEEDS TO BEEMPHASIZEDD. AbbreviationsEMM Equipment Maintenance Manual Y YearsMIL US Military Specification TSN Time Since NewHF HeliFab N/C No ChangeFH Flight HoursMo. Months1.2 GeneralA. Any special tools required will be defined in the text of this document.B. Torque Values. Unless specified otherwise, the following torques will be used.Size Type Torque10/32 Screws, nuts and bolts 20 to 25 in-lbs.1.3 Consumable MaterialsA. ExplanationConsumable materials are noted throughout this manual and on referenceddrawings, only those material are to be used. Helifab, the STC holder, can be contacted to approve alternate or equivalent materials.STC Holder:Helifab, Inc.1318 Smede Hwy.Broussard, La, 70518Telephone: 337-364-4357Web: www. CHAPTER 4 - AIRWORTHINESS LIMITATIONSThere are no overhauls, time limits, or airworthiness limitations associated with this type design change.The Airworthiness Limitations section is FAA approved and specifies inspections and other maintenance required under Secs. 43.16 and 91.403 of the Federal Aviation Regulations unless an alternative program has been FAA approved.CHAPTER 5 –INSPECTIONS5.1 Conditional Inspections after Operational IncidentsA. After any operational incident involving hard landings, sudden stoppage ofthe drive train or water immersion, the system must not be operated unlessall requirements and inspections are performed in accordancewith section 5.4 of this EMM.B. Notification of incident should be made to the STC holder for additionalrequirements.CHECKS5.2 Preflight Check - EquipmentNormal pre-flight inspections to be accomplished per Bell Helicopter rotorcraft flight manual.Pre-flight inspections may be performed by the pilot or qualified technician.5.3 100 Hour InspectionA. This inspection is to be performed by a qualified technician.B. In conjunction with this inspection a complete preflightinspection must be accomplished in accordance withSection 5.2.DATE:HELICOPTER S/N: REGISTRY NO:TOTAL TIME:5.4 12 Months/1200 Hour InspectionA. This check is to be accomplished by a qualified technician.B. In conjunction with this inspection a complete preflight and 100 hourinspection must be accomplished in accordance with Section 5.2 and 5.3 respectively. . DATE:HELICOPTER S/N: REGISTRY NO:TOTAL TIME:CHAPTER 6 – WEIGHT AND BALANCE6.1Weight and BalanceThe new empty weight and corresponding C.G. location must be determined and entered in the aircraftpermanent records.lowed installation locations. The CG of the mount in the primary installation location is at STA 118.9, RBL10.0; the CG of the mount in the alternate installation location is STA 118.9, RBL 8.7. The weight ofequipment mounted in the oxygen bottle mount must also be accounted for during weight and balance cal-culations.CHAPTER 35 – EMERGENGY MEDICAL SYSTEM 35.1 IntroductionThis section presents a description of the HeliFab PN HF-206-EMS-5001-1 oxygen bottle mount installa-tion in the 206L series helicopter. This section can be used as a familiarization and be developed into atraining program by users of the kit.35.2 General DescriptionThere is a primary and alternate installation location for the oxygen bottle mount. The primary location is shown in figure 1. The alternate location, shown in figure 2, is approximately 1.3 inches inboard of theprimary location. Both the primary and alternate installation locations use existing inserts for bottle mount attachment to the fuselage.The oxygen bottle mount consists of a mounting bracket, retention strap with latch, and a quick release pin.Two types of retention straps may be used for the bottle mount, one type (PN 5001-02) is constructed of all stainless steel material with a cam buckle for length adjustment and the second type (PN 5001-05) is con-structed of 1 inch nylon webbing with a cam buckle for length adjustment and a stainless steel attachment bracket.Table 1Parts List(1) Determine length of fastener upon installation unless otherwise specified.(2) Length of fasteners may be increased if necessary for proper engagement.(3) Torque all fasteners per torque table in section 1.2 of this document.(4) Item 1 may be replaced with item 9, 10, and 11. Item 9, 10 , and 11 are to be installed in eye of item 5 in order to secure item 3 to item 5.FIGURE 1Oxygen Bottle Mount Primary Installation LocationFIGURE 2 Oxygen Bottle Mount Alternate Installation LocationFIGURE 3Oxygen Bottle Mount InstallationSECTION B-BFIGURE 4 Array SECTION C-C(with PN 5001-02 Retaining Strap)FIGURE 4aSECTION C-C(with PN 5001-05 Retaining Strap)35.3 Removal/Installation and Troubleshooting –oxygen bottle mountA. References: Figures 1 thru 4aB. Special Tools NoneC. Consumables Materials: NoneD. Procedure:Removal(1)Remove items 1, 2, and 4 attaching the oxygen bottle mount to existing aircraft inserts.Installation(1)Install items 1, 2, and 4 attaching oxygen bottle mount to existing aircraftinserts. For torque values see Section 1.2.Troubleshooting(1)Loose or improper engagement of installation hardware can be corrected byreplacement of defective or worn parts.35.4 Removal/Installation - DecalA. References: Figures 3B. Special Tools NoneC. Consumables Materials: NoneD. Procedure:Removal(1) Remove item 7 decal P/N 5001-108.Installation(1) Install item 7 decal P/N 5001-108 in location shown on figure 3.35.5 CleaningA. References: NoneB. Special Tools: NoneC. Consumable Materials: Mild detergent or Bleach in 10 parts water to1 part bleach mixture.D. Procedure:1. Spray or brush cleaning solution on oxygen bottle mount2. Let stand 2 minutes then rinse thoroughly with fresh water.3. Allow to air dry thoroughly.35.6 RepairA. No repairs other than those that can be accomplished with normal methods or that are called out inthis EMM should be attempted unless the STC holder is contacted and specific written instructionsare provided. These instructions must be FAA approved or done in accordance with FAA accept-ed data specific to the repairs required.Normal repairs would include paint touch up and sheet metal repairs to nonstructural componentsdone in accordance with generally accepted maintenance practices.。